Approach to
Bleeding
Ryan Antel1
Published online: October 12, 2022
1Faculty of Medicine and Health Sciences, McGill University, Montreal, Canada
Corresponding Author: Ryan Antel, email ryan.antel@mail.mcgill.ca
DOI: 10.26443/mjm.v21i1.953
Abstract
This article presents a basic approach to the bleeding patient and is intended for medical students in their pre-clinical and clerkship years. Easy bruising and abnormal bleeding are relatively common symptoms, and may present as excessive bleeding post-injury, epistaxis, menorrhagia, prolonged bleeding after surgery or spontaneous bleeding. Identification and appropriate medical management of abnormal bleeding and bruising can decrease associated morbidity and mortality.
Tags: Bleeding, Coagulation, Hemostasis
Question
A 24-year-old women came to the urgent walk-in clinic due to continuous gum bleeding after a mouth injury the day prior. She was playing frisbee and was accidentally struck on her bottom lip when her friend threw the disc without warning. The patient had a history of bruising easily and of heavy menstruation with crampy lower abdominal pain. She had no significant medical history and took no medications other than occasional Advil. The patient did not use tobacco or illicit drugs, and had 2-3 alcohol drinks per week with her friends. She was a recreational athlete and ate a balanced diet. She reported that her mother also had a history of "bleeding issues." Her blood pressure was 117/73 mm Hg and her pulse was 78/min. Oropharyngeal examination showed blood oozing from a gum abrasion. A fading bruise was present on the left arm, but there were no other skin abnormalities. The remainder of the physical examination was normal. Initial investigation results were as follows:
Hemoglobin 107 (Normal: 120-160)
MCV 76 (Normal: 80-100)
Platelets 180,000 (Normal: 150,000-400,000)
PT 12 (Normal: 11-15)
INR 1 (Normal: < 1.1)
PTT 39 (24-32)
Which of the following conditions is most likely in this patient?
- Hemophilia A
- Von Willebrand Disease
- Hemophilia B
- Bernard-Soulier Syndrome
- Disseminated Intravascular Coagulation (DIC)
- Advanced liver cirrhosis
- Glanzmann’s Thrombasthenia
- Warfarin ingestion
Answer
B. This patient’s presentation is consistent with Von Willebrand Disease (VWD), an inherited disorder of platelet adhesion that is estimated to affect approximately 1% of the worldwide population. (1) Most individuals with VWD are asymptomatic, however, a minority of patients have easy bruising, skin bleeding and mucosal bleeding. (2) This patient’s normal platelet count effectively rules out a quantitative platelet disorder as a cause of her prolonged mucosal bleeding. As well, her normal PT and INR suggest that her extrinsic coagulation pathway is intact, making entities such as Warfarin ingestion (H), fat-soluble vitamin deficiencies and advanced liver cirrhosis unlikely (F). .
VWD is associated with impaired quality/quantity of von Willebrand factor (vWF), a glycoprotein produced by endothelial cells and platelets.(2) VWF contributes to platelet-endothelial binding and platelet aggregation, as well as acts as a carrier protein for coagulation factor VIII.(3) Therefore, individuals with VWD may have a slightly prolonged PTT due to degradation of factor VIII, as well as a prolonged bleeding time due to platelet dysfunction, however, PT/INR would typically be unaffected.(2)
A normal PTT would be expected in conditions such as Glanzmann’s Thrombasthenia (G) and Bernard-Soulier Syndrome (D), which are inherited platelets disorders.(4) Hemophilia tends to present with deep tissue bleeding, such as spontaneous hemarthroses, and is much more common in males than females (A,C).(5) Disseminated intravascular coagulation would be associated with a prolong PT and PTT due to coagulation factor consumption (E).(6)
Initial approach
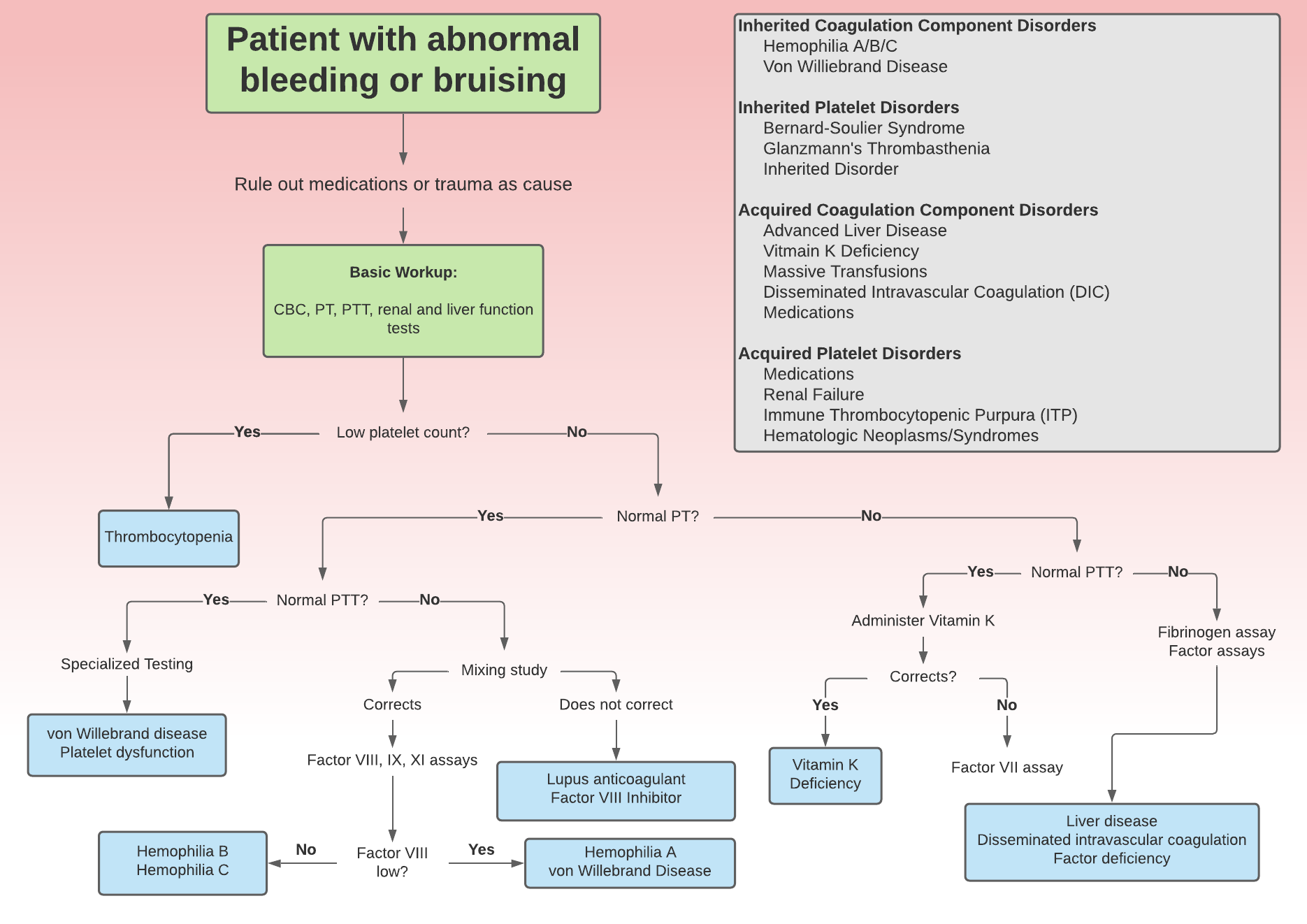
The initial investigation of a patient presenting with bleeding that is considered to be abnormal begins with a thorough history and physical exam. A detailed history should characterize the onset, course, duration, precipitating events and alleviating factors of the current bleeding episode. As well, inquiring about the presence of any constitutional symptoms (potentially concerning for malignancy), in addition to obtaining a detailed personal bleeding history, family history and medication history (with emphasis on anticoagulant and antiplatelet agents) is vital.(5) When assessing a patient with bleeding, symptoms such as pallor, dizziness and perceived tachycardia/palpitations may help characterize potential blood volume loss. In addition, a thorough physical examination may identify information regarding the origin and severity of the bleeding and can help tailor further investigations. Obtaining accurate vital signs along with an assessment of volume and perfusion status (such as checking distal pulses and capillary refill) is important for initial assessment. In addition, a complete head-to-toe examination is needed to identify any present signs of bleeding. For example, mucocutaneous bleeding (such as epistaxis, petechiae and gingival bleeding) tend to be more suggestive of platelet disorders.(4, 7) On the contrary, spontaneous hemarthroses, and muscle hemorrhages are commonly associated hemophilia or other congenital coagulopathies.(8) Initial laboratory evaluations should include a complete blood count, prothrombin time (PT) and partial prothrombin time (PTT) along with liver and renal function testing.(8) These basic laboratory parameters can indicate possible disorders affecting platelets and/or the clotting cascade, thus narrowing the differential diagnosis (Figure 1). A normal PT and PTT imply that the coagulation cascade is intact, suggesting a possible platelet source of bleeding.(8)
Prothrombin Time/International Normalized Ratio
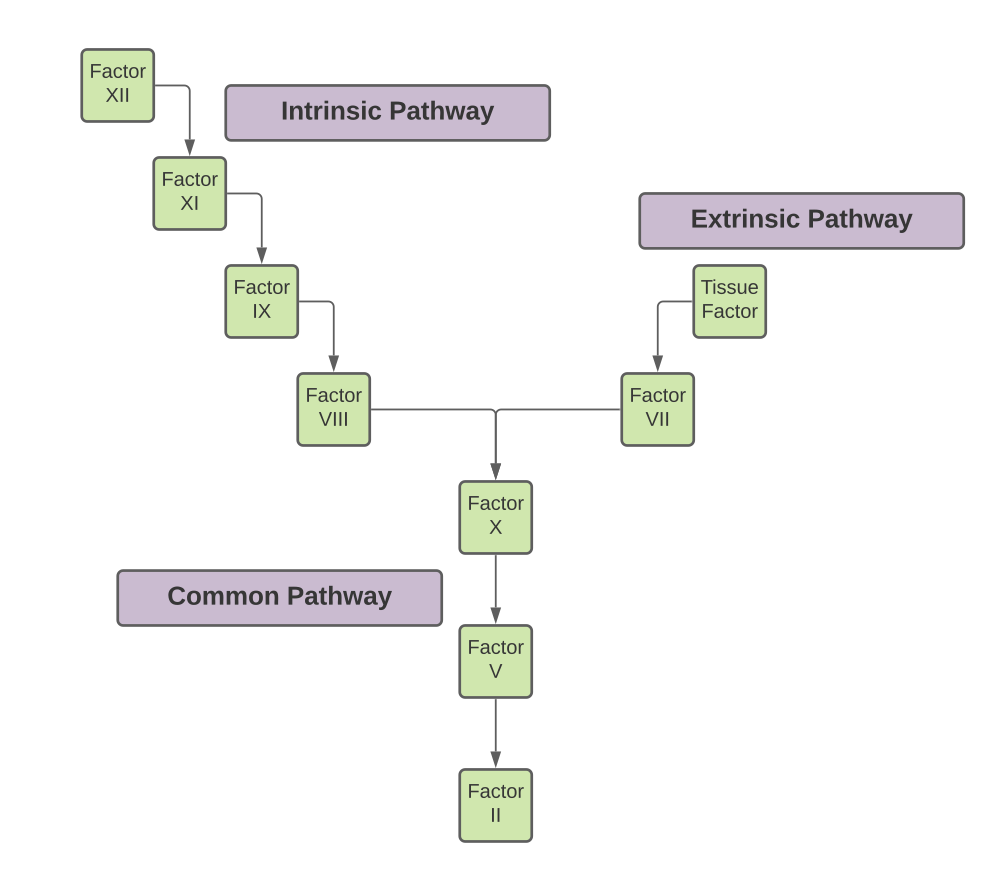
The prothrombin time (PT) is used to evaluate the function of the extrinsic and final common pathway of the coagulation cascade (Figure 2), with a prolonged PT indicating dysfunction of these processes.(9) As such, a prolonged PT may indicate deficiencies or inhibitors of clotting factors affecting the extrinsic and final common pathways.(10) A prolonged PT in the clinical setting is often due to the use of Warfarin, a commonly prescribed anticoagulant with effects on factor VII activity.(9) Other causes of PT prolongation include liver failure, vitamin K deficiency and consumptive coagulopathies (such as disseminated intravascular coagulation).(8) The International Normalized Ratio (INR) is often used in clinical practice to overcome interlaboratory variation within measured PT. It is a mathematical conversion of a patient’s measured PT that accounts for variability in reaction reagents used in the different laboratory settings.(8) As such, a prolonged INR should be interpreted as would a prolonged PT.
Partial Thromboplastin Time (PTT)
To evaluate the function of the intrinsic and final common pathway of the coagulation cascade, the partial thromboplastin time (PTT) is used (Figure 2).(9) Similarly to the PT, deficiencies or inhibitors of clotting factors within the extrinsic and final common pathways result in prolongation of the PTT.(8) In the clinical setting, PTT is used to monitor response to heparin, an anticoagulant that works on factors II (thrombin) and factor X.(10) Other causes of PTT prolongation include congenital factor deficiencies (such as in Hemophilia), decrease in certain coagulation factors (such as VIII in VWD) and consumptive coagulopathies (such as disseminated intravascular coagulation).(8, 11)
Other Laboratory Testing
When evaluating a patient with abnormal bleeding, it is often relevant to obtain complete blood counts, as well as renal and liver function tests, to evaluate for underlying systemic disease.(5) Peripheral blood smears may also be of value, to help confirm the presence/absence of thrombocytopenia as well as to evaluate platelet morphology and possible hematologic malignancies or hyperproliferation conditions.(8) Classically, measurement of a patient’s bleeding time was used to assess platelet function. This involved the creation of a standardized skin incision in order to record the time until bleeding ceased, however, this has now been mostly replaced with modern alternative platelet function analyzers.(12) Specialized laboratory testing for specific underlying causes of abnormal bleeding can be used once the differential diagnosis has been narrowed by the use of more preliminary measures as above. These specialized investigations include assays to assess specific coagulation factor titers (such as assays for factors VIII, IX or XI), procoagulant particle activity (von Willebrand factor antigen quantification/activity) or the presence of certain coagulation factor inhibitors (lupus anticoagulant detection).(10)
Beyond Initial Approach
In this section, commonly described causes of bleeding are discussed in more detail.
Inherited Disorders
Hemophilia A and B
Hemophilia A and B are inherited bleeding disorders due to the deficiency of factors VIII and IX, respectively.(5, 11) These disorders are passed along via an X-linked recessive inheritance pattern, as such, males are more affected than females.(8) Patients affected by these disorders can experience profound bleeding after surgeries as well as spontaneous bleeding into deep tissues (causing entities such as spontaneous hemarthroses).(5)
Von Willebrand Disease (VWD)
VWD is due to a defect in Von Willebrand factor (VWF), which is a glycoprotein produced by platelets which functions to facilitate platelet adhesion and aggregation.(3) VWF also acts as a carrier for factor VIII. As such, individuals with VWD may have a prolonged PTT.(10) Treatment for this disease is possible without the use of blood products, as tranexamic acid and desmopressin have been seen to be helpful in correcting bleeds.(3) However, when indicated, factor VIII concentrates and other plasma products can be used to overcome bleeding.(5)
Inherited Platelet Disorders
Several blood disorders associated with dysfunctional platelets can result in a bleeding diathesis due to defective primary hemostasis. Many inherited thrombocytopathies have been characterized and each can be classified into abnormal platelet adhesion, activation or aggregation, or a combination thereof.(4) While many of these disorders are quite rare, Bernard-Soulier syndrome (BSS) and Glanzmann’s thrombasthenia (GT) are more commonly described.(5) BSS is caused by a qualitative or quantitative defect of the GPIbIX/V complex on the platelet membrane, which often leads to a severe bleeding disorder.(13) This complex is the major receptor for VWF and mediates both platelet agglutination and adhesion.(4) Meanwhile, GT is caused by a qualitative or quantitative deficiency of the platelet GPIIb/GPIIIa complex, which can lead to severely impaired platelet aggregation.(13)
Acquired Disorders
Medications
Drug-induced bleeding disorders can be classified according the mechanisms in which they disrupt bleeding. This includes drugs that 1) are anticoagulants themselves 2) potentiate the action of other anticoagulants 3) inhibit the activity of platelets 4) decrease the quantity of circulating platelets and 5) increase vascular fragility.(4, 5, 8, 9, 13) Many commonly used medications are implicated in these processes, although medications most commonly associated with bleeding include anticoagulants (particularly warfarin, heparin, enoxaparin, dabigatran, apixaban, and rivaroxaban), anti-platelet agents (such as clopidogrel, aspirin), corticosteroids, and non-steroidal anti-inflammatory drugs.(8)
Disseminated Intravascular Coagulation
Disseminated intravascular coagulation (DIC) describes the widespread activation of coagulation, leading to intravascular formation of fibrin and thrombotic occlusion of small and midsize vessels.(6) The resulting depletion of platelets and coagulation proteins resulting from the ongoing coagulation can cause a severe bleeding diathesis.(8) As such, deep tissue bleeding is often the presenting symptom in a patient with DIC. DIC is associated with many clinical conditions, although bacterial infections, widespread trauma, malignancies, obstetrical complications, reactions to toxins and immunologic disorders are often implicated.(6, 14) The development of DIC is associated with unfavourable outcomes and has been shown to be an independent predictor of mortality.(6)
Acquired Platelet Disorders
Acquired platelet disorders are relatively common in clinical practice, in contrast to congenital platelet disorders.(4, 7) As discussed above, medications such as aspirin have well-documented anti-platelet activity and are commonly used in patients with cardiovascular disease. However, these patients may be at increased risk of subsequent bleeding. Other medications with possible anti-platelet activity include some anti-inflammatory drugs and herbal remedies.(8) Systemic medical disorders may also disrupt platelet quality/quantity including uremia due to renal failure, anti-platelet antibodies (in conditions such as systemic lupus erythematosus and idiopathic thrombocytopenic purpura) and hematologic disorders (such as myeloproliferative neoplasms, leukemias and myelodysplastic syndromes).(15) Other causes of acquired thrombocytopenia include viral infections (such as Epstein-Barr virus, parvovirus B19 and cytomegalovirus), autoimmune syndromes (such as idiopathic thrombocytopenic purpura) and splenic sequestration.(16)
Liver Disease
Individuals with liver disease can have a bleeding diathesis that is often multifactorial. The liver is responsible for synthesizing many of the major coagulation factors, which may be affected in states of liver pathology.(10) Reduced levels of factors II, V, VII, IX, X and XI are commonly seen in advanced liver failure.(5) As such, prolonged PT/INR and PTT can be seen.(10) In addition, liver disease can lead to thrombocytopenia, as portal hypertension contributes to the splenic sequestration of platelets.(5)
Vitamin K Deficiency
Several key coagulation factors (II, VII, IX, X) depend upon sufficient Vitamin K for carboxylation in the liver.(5) As such, a vitamin K deficiency (or intrinsically impaired carboxylase activity in the liver) may lead to ineffective coagulation.(8) This would prolong the PT/INR, much in the same way that administration of warfarin would. Oral or parental vitamin K should be given if deficiency is suspected.(5)
Massive Transfusion
Classically, a massive transfusion is defined as a replacement of a patient’s total blood volume in 24 hours.(5) As transfusions of packed red blood cells (PRBCs) do not contain significant levels of clotting factors and can cause a dilutional coagulopathic state, regular infusion of coagulation factors in the forms of fresh frozen plasma or platelet rich plasma should be given throughout the transfusion protocol.(17)
Conclusion
Easy bruising and abnormal bleeding are relatively common symptoms experienced by patients. Proper identification and appropriate medical management of such presentations can decrease associated morbidity and mortality. Physicians should be prepared with an approach to patients with abnormal bleeding to identify and address common underlying etiologies. Ultimately, the implementation of basic clinical method, guided by a thorough history and physical exam, is an essential starting point to initially asses such patients as well as to guide further investigations.
References
- Kurth AA, Ludwig G, Scharrer I. [Prevalence, pathophysiology, diagnosis and treatment of von Willebrand syndrome in orthopedic trauma patients]. Orthopade. 1999;28(4):366-74
- Ng C, Motto DG, Di Paola J. Diagnostic approach to von Willebrand disease. Blood. 2015;125(13):2029-37
- Swami A, Kaur V. von Willebrand Disease: A Concise Review and Update for the Practicing Physician. Clin Appl Thromb Hemost. 2017;23(8):900-10
- D'Andrea G, Chetta M, Margaglione M. Inherited platelet disorders: thrombocytopenias and thrombocytopathies. Blood Transfus. 2009;7(4):278-92
- Gopinath R, Sreekanth Y, Yadav M. Approach to bleeding patient. Indian J Anaesth. 2014;58(5):596-602
- Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341(8):586-92
- Perez Botero J, Di Paola J. Diagnostic approach to the patient with a suspected inherited platelet disorder: Who and how to test. J Thromb Haemost. 2021;19(9):2127-36
- Neutze D, Roque J. Clinical Evaluation of Bleeding and Bruising in Primary Care. Am Fam Physician. 2016;93(4):279-86
- Kruse-Jarres R, Singleton TC, Leissinger CA. Identification and basic management of bleeding disorders in adults. J Am Board Fam Med. 2014;27(4):549-64
- Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc. 2007;82(7):864-73
- Benson G, Auerswald G, Dolan G, Duffy A, Hermans C, Ljung R, et al. Diagnosis and care of patients with mild haemophilia: practical recommendations for clinical management. Blood Transfus. 2018;16(6):535-44
- Russeau AP, Vall H, Manna B. Bleeding Time. StatPearls. Treasure Island (FL)2022.
- Al-Huniti A, Kahr WH. Inherited Platelet Disorders: Diagnosis and Management. Transfus Med Rev. 2020;34(4):277-85
- Iba T, Levy JH. Sepsis-induced Coagulopathy and Disseminated Intravascular Coagulation. Anesthesiology. 2020;132(5):1238-45
- Casari C, Bergmeier W. Acquired platelet disorders. Thromb Res. 2016;141 Suppl 2:S73-5
- Greenberg EM, Kaled ES. Thrombocytopenia. Crit Care Nurs Clin North Am. 2013;25(4):427-34
- Abuzeid AM, O'Keeffe T. Review of massive transfusion protocols in the injured, bleeding patient. Curr Opin Crit Care. 2019;25(6):661-

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.