Narrative Review
The Interplay Between COVID-19 and Cardiovascular Disease
Brandon Shokoples1, Nathanne S. Ferreira1, Kevin Comeau1
Published online: October 18, 2021
1McGill University
Corresponding Author: Brandon Shokoples, email brandon.shokoples@mail.mcgill.ca
DOI: 10.26443/mjm.v20i2.880
Abstract
Introduction: The emergence of the global COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus (SARS-CoV-2) has created a substantial burden on healthcare systems worldwide resulting in over 4 million deaths. The systemic impacts of COVID-19 infection are severe and broad in their implications, and the cardiovascular system is no exception. The SARS-CoV-2 binds the angiotensin-converting enzyme-2 (ACE2) receptor to infect host cells, with ACE2 representing a critical regulator of blood pressure homeostasis and proper cardiovascular functioning.
Discussion: Patients with a history of cardiovascular disease are at an increased risk for hospitalization and mortality, and COVID-19 infection has now been demonstrated to initiate acute, but serious, episodes of cardiovascular events such as stroke or myocardial infarction (MI). As cases continue to rise around the world, understanding the interplay between COVID-19 infection and the cardiovascular system will be important for healthcare systems to adequately respond to the pandemic and prepare for future challenges.
Conclusion: Evidence suggests that COVID-19 infection can spur the onset of various cardiovascular pathologies, including thrombosis, stroke, arrhythmia, and MI, and these complications contribute to poorer patient outcomes. Patients presenting with symptoms of cardiovascular disease may also be foregoing medical treatment out of fear that is brought on by the pandemic or due to strain on healthcare systems preventing access to care. The direct physiological and indirect social consequences of COVID-19 will undoubtedly lead to further challenges for healthcare systems now and in the future.
Relevance: This paper discusses the deleterious cardiovascular consequences induced by the global COVID-19 pandemic.
Tags: COVID-19, Cardiovascular disease, Stroke, Myocardial infarction, Hypertension
Introduction
In December of 2019, the world was introduced to a novel coronavirus, coined the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is responsible for the Corona Virus Disease-19 (COVID-19) pandemic. Since its emergence in China, COVID-19 has escalated into a global pandemic infecting over 199 million people and resulting in over 4.2 million deaths worldwide. (1) As case counts continue to climb, it has become increasingly evident that patients with underlying cardiovascular disease have an increased risk of mortality and morbidity as a result of COVID-19 infection.
Cardiovascular disease encompasses a wide range of pathologies including heart disease and stroke and is reported by the World Health Organization as the leading cause of mortality worldwide. Clinical and epidemiological evidence presents a clear link between pre-existing cardiovascular disease and the severity of COVID-19 infection, as people with underlying cardiovascular disease appear to be more vulnerable to becoming severely ill and/or dying from the virus. (2) However, the association between infectious disease and cardiovascular disease is not a recent discovery. Over the last century clinicians have noted an increase in incidence of acute myocardial infarction (MI) during outbreaks of influenza. (3) In a cohort of 332 patients, the incidence of admissions for MI was six-times higher during the 7 days after confirmation of influenza infection than during the control interval (1 year pre-and post-confirmed influenza infection). (4) Other observational studies using clinical data have further confirmed an increased incidence ratio and increased odds for adverse cardiovascular reactions following lab-confirmed influenza infection in large cohorts of patients. (5, 6) While these studies give strong support for influenza’s role in increasing the likelihood of cardiovascular events, the pathological mechanisms underlying these observations have been reconsidered in the wake of the global COVID-19 pandemic.
Considering the rapid spread of COVID-19 across the world and the inability of countries to address and adequately respond to the effects of the pandemic, there is an increased need for understanding the interplay between COVID-19 infection and cardiovascular disease. This review will briefly introduce how COVID-19 interacts with the cardiovascular system, describe why patients with cardiovascular disease are at an increased risk of succumbing to COVID-19, and discuss what the long-term cardiovascular implications of COVID-19 infection could mean.
The Mechanisms Behind COVID-19 Infection and Relevance to the Cardiovascular System
COVID-19 infection is described as having three phases, beginning with mild upper respiratory syndrome, followed by a parenchymal pulmonary phase characterized by marked hypoxemia, and finally progression to a hyperinflammatory prothrombotic phase with multiorgan dysfunction and strong potential for thromboembolism. The substantial proinflammatory response to viral infections upregulates the frequency of immune cell subsets, such as macrophages, that induce a cytokine storm. Further systemic effects include increased expression of tissue factor, markers of thrombin generation and platelet activation, complement activation, and an increased risk of intravascular thrombosis. Whether, and to what degree, the clinically recognized cardiovascular manifestations of COVID-19 are a direct result of viral injury, prolonged hypoxemia, vascular endothelial cell infection/inflammation, cardiac pericyte infection, or intravascular thrombosis remains unknown. Vascular complications of COVID-19 have also been reported with evidence of viral particles within vascular endothelial cells and diffuse vascular endothelial cell injury associated with increases in inflammatory mediators of the lungs, heart, kidneys, and intestinal tissues that could culminate in thrombotic disease, stroke, arrhythmias, myocardial infarction, neurological manifestations including encephalopathy, acute respiratory distress syndrome, proteinuria, acute kidney injury, septic shock, and multiple organ failure (Figure 1). (7)
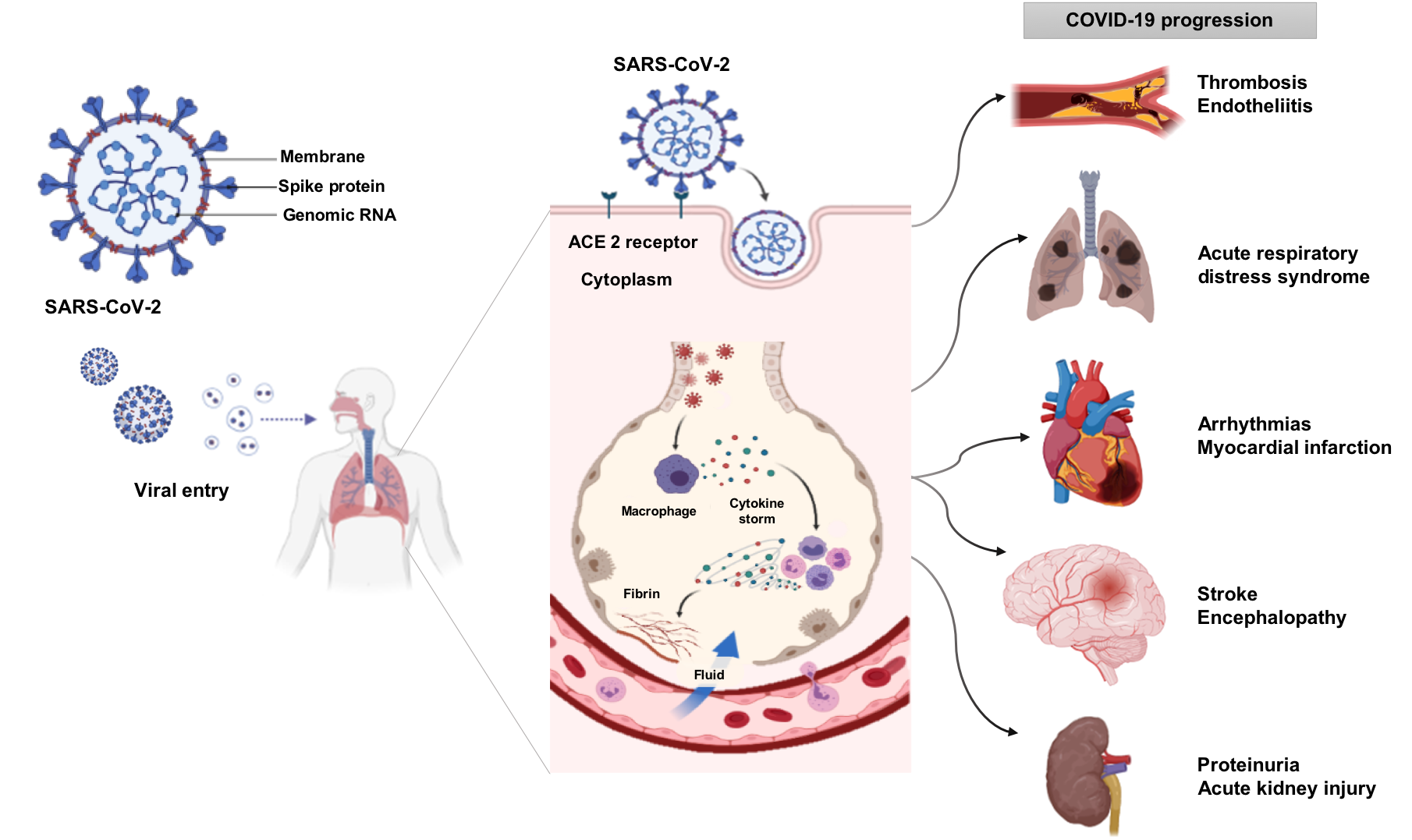
The coronavirus, SARS-CoV-2, that causes the disease, COVID-19, enters the cell using the ACE2 receptor. The principal point of entry is the upper respiratory tract. Viral entry into the cell induces inflammation in the respiratory tract, manifesting in progressive systemic inflammation with a cytokine storm. The increase of immune cells, cytokines, and inflammatory mediators affects several organs such as the heart, lung, brain and kidney. The progression of systemic inflammation can promote acute respiratory distress syndrome, acute kidney injury, thrombosis, stroke, arrhythmias, myocardial infarction, septic shock, and multiple organ failure.
Similar to the SARS-CoV-1 virus responsible for the 2002-2004 SARS epidemic, viral entry of SARS-CoV-2 occurs after proteolytic cleavage of the viral spike (S) protein upon binding to angiotensin-converting enzyme-2 (ACE2) receptor. This binding, in concert with S-protein priming by the host cell transmembrane protease serine 2 precursor (TMPRSS2), leads to host cell entry of the virus. Notably, SARS-CoV-2 is more pathogenic, at least in part due to its 10- to 20-fold increased binding affinity to ACE2 as compared to the SARS-CoV-1 virus (Figure 1). (8)The fact that SARS-CoV-2 utilizes ACE2 to gain entry into host cells provides strong evidence that COVID-19 infection can have a direct impact on the cardiovascular system. ACE2 is a key modulator of the renin-angiotensin-aldosterone-system (RAAS). The RAAS is a principal regulator of blood volume and blood pressure homeostasis; consequently, perturbations to RAAS signaling are at the root of many cardiovascular diseases like hypertension and heart failure. (9) ACE2 is positioned at a key intercept in this homeostatic process by virtue of being highly expressed in type II alveolar cells of the lung, cardiac myocytes, cardiac pericytes, and vascular endothelium, serving to convert the pro-hypertensive and pro-inflammatory peptide angiotensin (Ang) II to Ang 1–7. The effects of Ang 1-7 are multi-factorial and include vasodilatory, natriuretic, anti-inflammatory, and antioxidant effects. (10)
ACE2 expression levels are found to be increased in many cardiovascular diseases, possibly as a negative feedback mechanism to counter the effects of Ang II, and may help explain why the symptoms of COVID-19 appear to be more severe in patients with existing cardiovascular conditions. (11) Increased expression of ACE2 provides more potential docking sites for SARS-CoV-2 increasing the risk for initial infection. Subsequently, when the virus gains entry to the cell, ACE2 is internalized and thus no longer able to exert its anti-inflammatory effects. (12) Viral particles have been identified in endothelial cells providing a possible trigger for endothelial damage throughout the cardiovascular system. (13) Endothelial damage is linked with hypercoagulability and may explain the increased incidence of thrombosis and ischemic stroke seen with COVID-19 infection. (14, 15) Infection of kidney endothelial cells is speculated to contribute to acute kidney injury in patients with COVID-19 and may exacerbate pre-existing cardiovascular conditions. (15, 16)
Discussion
Deleterious Cardiovascular Manifestations of COVID-19 Infection
A concerning feature of COVID-19 is its unknown potential for long-term negative impact on cardiovascular health. A study released from Hong Kong demonstrated that 42.3% of COVID-19 survivors with non-severe disease and without overt cardiac manifestations displayed cardiac abnormalities up to four weeks post hospital discharge, with up to 8% displaying signs of myocardial injury. (17) The study was limited in patient number and in duration (only 4 weeks post infection); however, it raises concern for a potential future influx of patients with cardiovascular disease as the number of people who have been infected continues to rise. The substantial systemic inflammation induced by COVID-19 infection is likely to have a sizable impact on cardiovascular function, as the immune system is a known driver of many cardiovascular pathologies. The following sections will briefly describe what the most frequently reported cardiac manifestations of COVID-19 infection are and highlight the mechanisms for how COVID-19 could induce these poor cardiovascular outcomes.
Thrombotic Disorders
It is now well established that COVID-19 infection can induce a hypercoagulable state that can leave those infected at an increased risk of acute thrombotic events or coagulation abnormalities. (14) In fact, thrombotic events were reported by one study in up to 31% of the patients admitted to the ICU with a diagnosis of COVID-19. (18) The occurrence of thrombotic events in COVID-19 patients is often identified by elevated circulating D-dimer levels(19), which is a fibrin degradation product. (20) In COVID-19 patients, the degree of D-dimer elevation correlates strongly with mortality(19, 21) and may serve as an important prognostic tool for monitoring the severity of the infection. Temporal increases in D-dimer levels are a strong predictor for patient mortality, resulting in a critical diagnostic tool for early intensive medical interventions. (21, 22) Other biomarkers used to identify the occurrence of thrombosis include Von Willebrand Factor, fibrinogen, and P-selectin, all of which have consistently been found to be elevated in COVID-19, marking the progression of the disease. (23)
Several mechanisms have been proposed to explain how SARS-CoV-2 induces this hypercoagulable state, including endothelial injury(24) and increased inflammatory, and prothrombotic factors. (22, 25) Pro-inflammatory cytokines such as interleukin (IL)-6, IL-1ß, and interferon-? have been reported to be significantly elevated in COVID-19 patients. (25) These cytokines are known to damage endothelial cells, and this endothelial injury can trigger pro-thrombotic cellular cascades. Endothelial cells also highly express ACE2; therefore, they are more susceptible to infection with SARS-CoV-2. (13) Once infected, endothelial cells can enter in an inflammatory state of cellular death, termed pyroptosis, triggering further immune activation, inflammation, and subsequent thrombosis. Because the occurrence of thrombotic events in COVID-19 patients has been associated with poorer patient outcomes,(26) several clinical trials have emerged to identify the efficacy of anti-coagulant therapies in the treatment of COVID-19 patients. (reviewed by (27))
Stroke
In April 2020, it was estimated that as many as 4.9% of patients with COVID-19 had an episode of acute ischemic stroke during initial hospitalization. (28) As more data has been collected, it appears that stroke actually occurs in closer to 1-2.7% of COVID-19 patients. (29-32) However, whether SARS-CoV-2 infection contributes to the development of stroke is still controversial. A study of 14,483 patients infected with COVID-19 found a stroke prevalence of 1.1%, of which 42.6% were of cryptogenic in etiology. (32) The authors, along with several other groups, suggest that the hypercoagulability state associated with elevated D-dimer could be an underlying cause for the increased proportion of cryptogenic stroke in patients; consequently, COVID-19 could represent a novel stroke mechanism. (32-34) However, a more recent study looking at a database of 27,676 patients, 8 163 of which had confirmed COVID-19, found that acute stroke occurred in only 1.3% of infected patients, compared to 1% without COVID-19. This implies that COVID-19 did not significantly influence the occurrence of acute stroke. (31) Similarly, a smaller study from Italy did not find an association between COVID-19 infection and stroke incidence. (35) Notably, neither of the aforementioned studies investigated the incidence of cryptogenic stroke. Therefore, we cannot exclude the possibility that COVID-19 could influence the incidence of cryptogenic stroke. Further investigation is required to determine whether COVID-19 infection influences stroke occurrence.
Arrhythmias
Arrhythmias, or alterations to the rhythm of the heart, have been one of the most common pathological cardiac manifestation of COVID-19 infection. A global study consisting of 4,526 patients from 12 countries and 4 continents found that approximately 18% of COVID-19 patients developed some form of arrythmia. (36) Even more concerning, the authors found that 40% of patients with arrhythmia needed to be mechanically ventilated, and only half survived. Another recent publication from Hong Kong followed patients with uncomplicated COVID-19 infection for up to 4 weeks post hospital discharge and found that 28% of patients developed an arrhythmia. (17) The mechanistic details for how SARS-CoV-2 can induce arrhythmia is still being deciphered, but there is emerging evidence that SARS-CoV-2 can directly infect cardiomyocytes and induce acute myocarditis, which can lead to the development of arrhythmias. (37, 38)
Interestingly, a 100-day observational study of 5,963 patients in the United States found that the incidence of ventricular arrhythmias decreased as the pandemic progressed, with the largest percent decrease in the states also with the highest COVID-19 case counts (up to 39% decrease in incidence). (39) In a subpopulation of 2,458 patients that had been monitored before the onset of the pandemic as well as during the pandemic, there was a significant reduction in ventricular arrhythmias. The authors speculated that this decline in the frequency of arrhythmia was due to a reduction in factors that favour or trigger arrhythmia, including reduced workplace and/or social stressors while working from home as well as decreased physical stressors induced by vigorous exercise. However, the authors remain uncertain about the exact reasons for the decreased incidence of arrythmia. It appears that COVID-19 infection can trigger the development of arrhythmia in infected individuals, but the social and workplace changes set in place for the pandemic may actually be decreasing the overall occurrence of arrhythmia in the general population. It will be interesting to follow future studies on the issue to see how the incidence of arrhythmias change as the pandemic progresses and safety protocols are lifted across the globe.
Myocardial Infarction
In early 2020 the cardiovascular complications associated with COVID-19 became much more apparent, and studies finding an increased risk for MI associated with COVID-19 infection began to appear in the literature. (40) A study of 5,119 Danish COVID-19 patients found that the instance of acute MI was approximately 5 times higher in the 14 days following COVID-19 diagnosis when compared to the 180 days before COVID-19 diagnosis. (41) A study across 4 hospital sites in New York City also found significant increases in the hazard ratio for arterial thrombotic events in COVID-19 patients, with MI being included under the umbrella of these events. (26) When looking at patients 45 years or older, the overall hazard ratio for arterial thrombotic events ranged from 1.65 to 2.71 with the highest hazard ratio associated with the oldest cohort (75 years old and up). In Sweden, a study investigating 1,946 cases of out-of-hospital cardiac arrest (OHCA) and 1,080 cases of in-hospital cardiac arrest (IHCA), found that 10% of all OHCA and 16% of all IHCA patients had COVID-19. (42) Patients with a confirmed COVID-19 diagnosis had a 3.4-fold (OHCA) and 2.3-fold (IHCA) increased risk of 30 day mortality in comparison to non-COVID-19 infected individuals.
In contrast to the information presented above, many hospital sites have actually seen a lower instance of MI than what would have be observed before the COVID-19 pandemic. In Italy, researchers found a significant reduction in admissions for MI across the country when they compared the week of March 12 to 19 in 2020 with that of 2019. (43) Surprisingly, a reduction of nearly 50% in MI admissions was observed from 2019 to 2020. A similar study looking at patients admitted for MI in Austria found that over the course of March 2 to 29, 2020, there was a nearly 40% decline in hospital admissions and in medical interventions for acute coronary syndrome and MI. (44) This may sound like a sliver of positivity in the midst of the pandemic; however, investigations into excess deaths due to COVID-19 paint a more sombre picture.
While the available literature presents a surplus of theory and speculation about hypothesized increases in the instance of MI with COVID-19 infection, there is relatively limited clinical evidence for increased MI in patients with a confirmed COVID-19 diagnosis. In theory, both type 1 and/or type 2 MI could be precipitated by COVID-19 infection. Type 1 MI is generally characterized by plaque rupture and thrombus formation(45), and type 1 MI could be induced by systemic inflammatory stress as a result of COVID-19, leading to plaque instability and rupture. (46) In the case of type 2 MI, hypoxic respiratory failure along with fever, tachycardia, and endocrine dysfunction, as a result of infection, may lead to an imbalance between cardiac oxygen supply and demand. (47) Both of these outcomes have a relatively poor prognosis without immediate medical intervention, and as more clinical data becomes available, it will be possible to better understand the cardiovascular risks associated with COVID-19. At the moment, our understanding of the real-world association between MI and COVID-19 infection remains limited to the data that has been released.
Cardiovascular Disease and the Link with COVID-19 Mortality
Cardiovascular disease is one of the most frequently associated co-morbidities of COVID-19 infection, and it has been linked with a significant increase in the risk for mortality. (48) A summary of studies around the world that present data pertaining to the incidence of cardiovascular disease and COVID-19 mortality is presented in Table 1. In general, it appears that patients with cardiovascular disease who become infected are more likely to end up in the intensive care unit (ICU), and they are also at an increased risk for mortality, regardless of their country of residence. A global study found that the proportion of the population with cardiovascular disease significantly correlated with that same country’s COVID-19 case fatality rate. (49) It was found that for every 1% increase in a country’s incidence of cardiovascular disease, the death rate from COVID-19 was 19% higher.
| Table 1: Prevalence of comorbid cardiovascular disease upon admission and its effect on mortality | Country | Total Number of Patients | Hypertension | Other CVD (%) | Intensive Care (% of those with HTN) | Intensive Care (% of those with CVD) | Intensive Care (% Total population) | Mortality (% of those with HTN) | Mortality (% of those with CVD) | Mortality (% of total) | Reference |
| Worldwide | 4526 | 2499 (55.2) | 827* (18.3) | N/A | 358 (43.4)* | N/A | N/A | 403 (48.7)* | 403 (48.7)* | (36) |
| Australia | 103 | 37 (36) | 16 (16) | 11 (29.7) | 6 (37.5) | 18 (17.5) | 4 (10.8) | 3 (18.8) | 4 (3.9) | (63) |
| Canada | 811 | 361 (44.5) | 171 (21.1) | 149 (41.3) | 67 (39.2) | 328 (40.4) | 97 (26.9) | N/A | 166 (20.5) | (64) |
| China | 1099 | 165 (15) | 42 (3.8)† | 24 (14.5)‡ | 10 (23.8)†, ‡ | 55 (5.0) | N/A | N/A | 15 (1.4) | (65) |
| China | 157 | 88 (56.0) | 25 (16.0)§ | N/A | N/A | N/A | 22 (25.0) | 12 (48.0) § | 37 (23.6) | (50) |
| China | 191 | 58 (30) | 15 (8) | N/A | N/A | N/A | 26 (44.8) | 13 (86.7) | 54 (28.3) | (22) |
| Italy | 1591 | 509 (32.0) | 223 (14.0) | 504 (100) || | 732 (100) || | 1591 (100) || | 195 (38.7) | N/A | 405 (25.5) | (66) |
| Netherlands | 952 | 374 (39.3) | 184 (19.3) | 195 (52.1)** | 95 (51.6) ** | 476 (50.0) ** | N/A | N/A | 239 (25.1) | (67) |
| Spain | 2070 | 919 (44.6) | 324 (15.8) | N/A | N/A | N/A | 255 (27.7) | 102 (31.5) | 393 (19.0) | (68) |
| USA | 5700 | 3026 (53.1) | 966 (35.8) †† | N/A | 228 (16.7) | 373 (6.5) | 384 (12.7) | (N/A) | 291 (9.7) | (51) |
| USA | 393 | 197 (50.1) | 82 (20.9) ‡‡ | 70 (35.5) | 31 (37.8) | 130 (33.1) | (N/A) | (N/A) | 40 (10.1) | (69) |
| USA | 586 | 353 (60.2) | 215 (36.7) | N/A | N/A | 196 (33.4) | 60 (17.0) | 105 (27.9) §§ | 82 (14.0) | (48) |
*Prevalence with an arrhythmia
† Prevalence of coronary heart disease and cerebrovascular disease combined.
‡ Numbers represent prevalence of composite endpoints: admission to intensive care unit, mechanical ventilation or death.
§ Based off of number of patients with arrythmia and cerebrovascular disease combined.
|| All patients in the study were admitted to the ICU. ||
920 patients were still hospitalized at the time of publication, therefore only the total number of patients that were either discharged or who had died were used for calculating %.
**Patients categorized as severely ill.
†† Prevalence of coronary artery disease and congestive heart failure combined.
The most prevalent comorbidity with COVID-19 upon hospital admission is hypertension (30-55% of COVID-19 patients, Table 1). The presence of elevated systolic blood pressure upon hospital admission was a strong predictor for the severity of respiratory distress and overall patient mortality. (50) Early in the pandemic, there was controversy as to whether or not the usage of angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs) should be discontinued in patients with COVID-19, as there was fear over the effects these commonly prescribed anti-hypertensives could have on ACE2 expression, and in turn, COVID-19 infection rates. (12) However, several studies have since emerged assuaging this concern, reporting no significant differences in hospitalization, length of stay, or mortality when these medications were administered. (48, 51-53) Interestingly, a recent observational study found that the transmission rate of COVID-19 to household contacts was actually lower in patients being treated with ACEI or ARB; however, caution must be observed in assuming a causal relationship. (54) Currently, the American Heart Association supports the continued use of RAAS inhibitors for the management of blood pressure in patients infected with SARS-CoV-2. (55)
COVID-19 and Ramifications for Cardiovascular Disease Patient Care
Beyond the numbers of lives lost that were directly attributed to COVID-19 infection, there remains a potential for cataclysm in the backlog of patients from an overstrained health care system. During the first and second waves of the COVID-19 pandemic in Canada, many hospitals forewent non-urgent procedures to reduce the burden on an already overstrained health care system. A study conducted in Ontario, Canada, showed that more than 1,200 medical procedures, including coronary artery bypass graft, angioplasty, and valve surgery, were postponed every month due to the pandemic. (49) This study revealed the serious disruption to essential health care services that were needed by patients living with cardiovascular disease.
In addition to decreased access to crucial medical services for patients, there may also be hesitancy on the part of patients to seek out medical attention due to social distancing and/or concerns of contracting COVID-19 in the hospital setting. (56) Consequently, it seems that many patients are not presenting themselves to health centres when experiencing mild symptoms of cardiovascular trouble out of anxiety or fear. (57) This phenomenon is ultimately leading to more deaths outside of the clinical setting and contributing to excess deaths not directly attributed to COVID-19. While hospitalization rates for MI have decreased in the United States and Europe, fatality rates for patients hospitalized for acute MI have increased. (58) This could be the result of patients who are experiencing mild symptoms avoiding medical care, while patients with severe symptoms seek out treatment. In turn, only the more severe cases would be treated in the clinical setting, and these patients are less likely to survive, increasing fatality rates. In addition, the patients experiencing mild symptoms may develop more severe symptoms later, leading to a poorer prognosis when they eventually present to the clinic. Speculation exists that the rates of hospital admissions for cardiovascular events will have a sharp increase over the coming months and years, as the patients who previously ignored their symptoms will be forced to access the treatment they initially avoided. This phenomenon is separate from the potential long-term cardiovascular complications that may arise from COVID-19 itself. (59) Taking into account (i) the increase in sedentary lifestyle as a result of large-scale lockdowns, (ii) the decrease in hospital visits for non-life threatening conditions due to disruptions in treatment from COVID-19, and (iii) the drug shortages paired with a lack of financial stability leading to fewer prescription refills, the occurrence of severe cardiovascular events has the potential to see a dramatic rise over the coming years. (60-62)
Conclusion
The COVID-19 pandemic has had a devastating toll on health care systems worldwide. Patients with underlying cardiovascular disease are at an increased risk for hospitalization, admission to the ICU, and mortality when compared to the general population. In addition, accumulating evidence suggests that COVID-19 infection itself can induce the onset of various cardiovascular manifestations, including thrombosis, stroke, arrhythmia, and MI. It is clear that these complications contribute to worse outcomes for patients suffering from COVID-19. Finally, patients with overt cardiovascular complications may be foregoing medical interventions out of fear or anxiety elicited from the COVID-19 pandemic. Taken together, the COVID-19 pandemic has direct and indirect effects on the occurrence and management of cardiovascular disease, and it is a matter to be taken seriously by both clinicians and the research community alike.
Acknowledgements
The work of the authors was supported by the Fonds de recherche Santé Quebec (FRQS) bourse 289184, Lady Davis Institute/TD Bank Studentship award and Canadian Institutes of Health Research (CIHR) Canada Graduate Scholarship to BGS and the McGill Department of Medicine Gordon Phillips Fellowship to KC. We would also like to thank Dr. Antoine Caillon for providing critical feedback during the editing process. Figures were created with BioRender.com.
References
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infectious Diseases. 2020;20(5):533-4. DOI:10.1016/s1473-3099(20)30120-1
- Mafham MM, Spata E, Goldacre R, Gair D, Curnow P, Bray M, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396(10248):381-9. DOI:10.1016/S0140-6736(20)31356-8
- Bainton D, Jones GR, Hole D. Influenza and Ischaemic Heart Disease-a Possible Trigger for Acute Myocardial Infarction? Int J Epidemiol. 1978;7(3):231-9. DOI:10.1093/ije/7.3.231
- Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N Engl J Med. 2018;378(4):345-53. DOI:10.1056/NEJMoa1702090
- Madjid M, Miller CC, Zarubaev VV, Marinich IG, Kiselev OI, Lobzin YV, et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34 892 subjects. European Heart Journal. 2007;28(10):1205-10. DOI:10.1093/eurheartj/ehm035
- Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611-8. DOI:10.1056/NEJMoa041747
- Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovascular Research. 2020;116(10):1666-87. DOI:10.1093/cvr/cvaa106
- Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251(3):228-48. DOI:10.1002/path.5471
- Paz Ocaranza M, Riquelme JA, García L, Jalil JE, Chiong M, Santos RAS, et al. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nature Reviews Cardiology. 2020;17(2):116-29. DOI:10.1038/s41569-019-0244-8
- Ferreira NS, Tostes RC, Paradis P, Schiffrin EL. Aldosterone, Inflammation, Immune System and Hypertension. Am J Hypertens. 2020. DOI:10.1093/ajh/hpaa137
- Louise, Stephen, Velkoska E, Sheila. The ACE2 gene: its potential as a functional candidate for cardiovascular disease. Clinical Science. 2013;124(2):65-76. DOI:10.1042/cs20120269
- Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. 2020;382(17):1653-9. DOI:10.1056/NEJMsr2005760
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020;395(10234):1417-8. DOI:10.1016/s0140-6736(20)30937-5
- Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950-73. DOI:10.1016/j.jacc.2020.04.031
- Romero-Sanchez CM, Diaz-Maroto I, Fernandez-Diaz E, Sanchez-Larsen A, Layos-Romero A, Garcia-Garcia J, et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95(8):e1060-e70. DOI:10.1212/WNL.0000000000009937
- Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. Acute Kidney Injury in COVID-19: Emerging Evidence of a Distinct Pathophysiology. J Am Soc Nephrol. 2020;31(7):1380-3. DOI:10.1681/ASN.2020040419
- Zhou M, Wong C-K, Un K-C, Lau Y-M, Lee JC-Y, Tam FC-C, et al. Cardiovascular sequalae in uncomplicated COVID-19 survivors. PLOS ONE. 2021;16(2):e0246732. DOI:10.1371/journal.pone.0246732
- Klok FA, Kruip MJHA, Van Der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research. 2020;191:145-7. DOI:10.1016/j.thromres.2020.04.013
- Naymagon L, Zubizarreta N, Feld J, Van Gerwen M, Alsen M, Thibaud S, et al. Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19. Thrombosis Research. 2020;196:99-105. DOI:10.1016/j.thromres.2020.08.032
- Rostami M, Mansouritorghabeh H. D-dimer level in COVID-19 infection: a systematic review. Expert Review of Hematology. 2020;13(11):1265-75. DOI:10.1080/17474086.2020.1831383
- Mueller C, Giannitsis E, Jaffe AS, Huber K, Mair J, Cullen L, et al. Cardiovascular biomarkers in patients with COVID-19. European Heart Journal Acute Cardiovascular Care. 2021. DOI:10.1093/ehjacc/zuab009
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-62. DOI:10.1016/S0140-6736(20)30566-3
- Grobler C, Maphumulo SC, Grobbelaar LM, Bredenkamp JC, Laubscher GJ, Lourens PJ, et al. Covid-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. International Journal of Molecular Sciences. 2020;21(14):5168. DOI:10.3390/ijms21145168
- Kidde J, Gorabi AM, Jamialahmadi T, Sahebkar A. COVID-19 Is an Endothelial Disease: Implications of Nitric Oxide. Springer International Publishing; 2021. p. 109-13.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. DOI:10.1016/S0140-6736(20)30183-5
- Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA. 2020;324(8):799-801. DOI:10.1001/jama.2020.13372
- McFadyen JD, Stevens H, Peter K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ Res. 2020;127(4):571-87. DOI:10.1161/CIRCRESAHA.120.317447
- Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke and Vascular Neurology. 2020;5(3):279-84. DOI:10.1136/svn-2020-000431
- Qin C, Zhou L, Hu Z, Yang S, Zhang S, Chen M, et al. Clinical Characteristics and Outcomes of COVID-19 Patients With a History of Stroke in Wuhan, China. Stroke. 2020;51(7):2219-23. DOI:10.1161/strokeaha.120.030365
- Katsanos AH, Palaiodimou L, Zand R, Yaghi S, Kamel H, Navi BB, et al. The Impact of SARS-CoV -2 on Stroke Epidemiology and Care: A Meta-Analysis. Annals of Neurology. 2021;89(2):380-8. DOI:10.1002/ana.25967
- Qureshi AI, Baskett WI, Huang W, Shyu D, Myers D, Raju M, et al. Acute Ischemic Stroke and COVID-19: An Analysis of 27 676 Patients. Stroke. 2021:STROKEAHA120031786. DOI:10.1161/STROKEAHA.120.031786
- Ramos-Araque ME, Siegler JE, Ribo M, Requena M, López C, De Lera M, et al. Stroke etiologies in patients with COVID-19: the SVIN COVID-19 multinational registry. BMC Neurology. 2021;21(1). DOI:10.1186/s12883-021-02075-1
- Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. Characteristics of ischaemic stroke associated with COVID-19. Journal of Neurology, Neurosurgery & Psychiatry. 2020;91(8):889-91. DOI:10.1136/jnnp-2020-323586
- Hernández-Fernández F, Sandoval Valencia H, Barbella-Aponte RA, Collado-Jiménez R, Ayo-Martín Ó, Barrena C, et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143(10):3089-103. DOI:10.1093/brain/awaa239
- Benussi A, Pilotto A, Premi E, Libri I, Giunta M, Agosti C, et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020;95(7):e910-e20. DOI:10.1212/wnl.0000000000009848
- Coromilas EJ, Kochav S, Goldenthal I, Biviano A, Garan H, Goldbarg S, et al. Worldwide Survey of COVID-19 Associated Arrhythmias. Circulation: Arrhythmia and Electrophysiology. 2021. DOI:10.1161/circep.120.009458
- Luetkens JA, Isaak A, Zimmer S, Nattermann J, Sprinkart AM, Boesecke C, et al. Diffuse Myocardial Inflammation in COVID-19 Associated Myocarditis Detected by Multiparametric Cardiac Magnetic Resonance Imaging. Circulation: Cardiovascular Imaging. 2020;13(5). DOI:10.1161/circimaging.120.010897
- Bojkova D, Wagner JUG, Shumliakivska M, Aslan GS, Saleem U, Hansen A, et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovascular Research. 2020;116(14):2207-15. DOI:10.1093/cvr/cvaa267
- O’Shea CJ, Thomas G, Middeldorp ME, Harper C, Elliott AD, Ray N, et al. Ventricular arrhythmia burden during the coronavirus disease 2019 (COVID-19) pandemic. European Heart Journal. 2021;42(5):520-8. DOI:10.1093/eurheartj/ehaa893
- Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, et al. ST-Segment Elevation in Patients with Covid-19 — A Case Series. N Engl J Med. 2020. DOI:10.1056/NEJMc2009020
- Modin D, Claggett B, Sindet-Pedersen C, Lassen MCH, Skaarup KG, Jensen JUS, et al. Acute COVID-19 and the Incidence of Ischemic Stroke and Acute Myocardial Infarction. Circulation. 2020;142(21):2080-2. DOI:10.1161/CIRCULATIONAHA.120.050809
- Sultanian P, Lundgren P, Stromsoe A, Aune S, Bergstrom G, Hagberg E, et al. Cardiac arrest in COVID-19: characteristics and outcomes of in- and out-of-hospital cardiac arrest. A report from the Swedish Registry for Cardiopulmonary Resuscitation. Eur Heart J. 2021. DOI:10.1093/eurheartj/ehaa1067
- De Rosa S, Spaccarotella C, Basso C, Calabrò MP, Curcio A, Filardi PP, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. European Heart Journal. 2020. DOI:10.1093/eurheartj/ehaa409
- Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. European Heart Journal. 2020. DOI:10.1093/eurheartj/ehaa314
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018). Journal of the American College of Cardiology. 2018;72(18):2231-64. DOI:10.1016/j.jacc.2018.08.1038
- Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of Coronavirus Disease 2019 (COVID-19) With Myocardial Injury and Mortality. JAMA Cardiology. 2020;5(7):751-3. DOI:10.1001/jamacardio.2020.1105
- Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. DOI:10.1136/bmj.m1091
- Pareek M, Singh A, Vadlamani L, Eder M, Pacor J, Park J, et al. Relation of Cardiovascular Risk Factors to Mortality and Cardiovascular Events in Hospitalized Patients with Coronavirus Disease 2019 (From the Yale COVID-19 Cardiovascular Registry). Am J Cardiol. 2021. DOI:10.1016/j.amjcard.2021.01.029
- Botly LCP, Martin-Rhee M, Kasiban A, Swartz RH, Mulvagh SL, Lindsay MP, et al. COVID-19 Pandemic: Global Impact and Potential Implications for Cardiovascular Disease in Canada. CJC Open. 2020;2(4):265-72. DOI:10.1016/j.cjco.2020.06.003
- Caillon A, Zhao K, Klein KO, Greenwood C, Lu Z, Paradis P, et al. High systolic blood pressure at hospital admission is an important risk factor in models predicting outcome of COVID-19 patients. Am J Hypertens. 2021. DOI:10.1093/ajh/hpaa225
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052. DOI:10.1001/jama.2020.6775
- Nouri-Vaskeh M, Kalami N, Zand R, Soroureddin Z, Varshochi M, Ansarin K, et al. Comparison of Losartan and Amlodipine Effects on the Outcomes of Patient with COVID-19 and Primary Hypertension: A Randomized Clinical Trial. International Journal of Clinical Practice. 2021. DOI:10.1111/ijcp.14124
- Khera R, Clark C, Lu Y, Guo Y, Ren S, Truax B, et al. Association of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers with the Risk of Hospitalization and Death in Hypertensive Patients with Coronavirus Disease-19. Journal of the American Heart Association. 2021. DOI:10.1161/jaha.120.018086
- Armstrong K, Soltoff A, Rieu-Werden M, Metlay J, Haas J. Use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers associated with lower risk of COVID-19 in household contacts. PLOS ONE. 2021;16(3):e0247548. DOI:10.1371/journal.pone.0247548
- HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19 [press release]. 2020.
- Garcia S, Albaghdadi Mazen S, Meraj Perwaiz M, Schmidt C, Garberich R, Jaffer Farouc A, et al. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States During COVID-19 Pandemic. Journal of the American College of Cardiology. 2020;75(22):2871-2. DOI:10.1016/j.jacc.2020.04.011
- Hammad TA, Parikh M, Tashtish N, Lowry CM, Gorbey D, Forouzandeh F, et al. Impact of COVID-19 pandemic on ST-elevation myocardial infarction in a non-COVID-19 epicenter. Catheterization and Cardiovascular Interventions. 2021;97(2):208-14. DOI:https://doi.org/10.1002/ccd.28997
- Gluckman TJ, Wilson MA, Chiu S-T, Penny BW, Chepuri VB, Waggoner JW, et al. Case Rates, Treatment Approaches, and Outcomes in Acute Myocardial Infarction During the Coronavirus Disease 2019 Pandemic. JAMA Cardiology. 2020;5(12):1419. DOI:10.1001/jamacardio.2020.3629
- Becker RC. Anticipating the long-term cardiovascular effects of COVID-19. J Thromb Thrombolysis. 2020:1-13. DOI:10.1007/s11239-020-02266-6
- Zheng C, Huang WY, Sheridan S, Sit CH-P, Chen X-K, Wong SH-S. COVID-19 Pandemic Brings a Sedentary Lifestyle in Young Adults: A Cross-Sectional and Longitudinal Study. Int J Environ Res Public Health. 2020;17(17). DOI:10.3390/ijerph17176035
- Rossen LM, Branum AM, Ahmad FB, Sutton P, Anderson RN. Excess Deaths Associated with COVID-19, by Age and Race and Ethnicity — United States, January 26–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1522-7. DOI:10.15585/mmwr.mm6942e2
- Badreldin HA, Atallah B. Global drug shortages due to COVID-19: Impact on patient care and mitigation strategies. Res Social Adm Pharm. 2021;17(1):1946-9. DOI:10.1016/j.sapharm.2020.05.017
- Toh DJW, Rowe E, Nelson R, O'Connell A, Lim K, Fielke L, et al. Outcomes for the first wave of hospitalised patients with COVID-19 in the South Australian context: a retrospective audit. Internal Medicine Journal. 2021;51(2):189-98. DOI:10.1111/imj.15106
- Murthy S, Archambault PM, Atique A, Carrier FM, Cheng MP, Codan C, et al. Characteristics and outcomes of patients with COVID-19 admitted to hospital and intensive care in the first phase of the pandemic in Canada: a national cohort study. CMAJ Open. 2021;9(1):E181-E8. DOI:10.9778/cmajo.20200250
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-20. DOI:10.1056/NEJMoa2002032
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574-81. DOI:10.1001/jama.2020.5394
- Pouw N, Van De Maat J, Veerman K, Ten Oever J, Janssen N, Abbink E, et al. Clinical characteristics and outcomes of 952 hospitalized COVID-19 patients in The Netherlands: A retrospective cohort study. PLOS ONE. 2021;16(3):e0248713. DOI:10.1371/journal.pone.0248713
- Velasco-Rodríguez D, Alonso-Dominguez J-M, Vidal Laso R, Lainez-González D, García-Raso A, Martín-Herrero S, et al. Development and validation of a predictive model of in-hospital mortality in COVID-19 patients. PLOS ONE. 2021;16(3):e0247676. DOI:10.1371/journal.pone.0247676
- Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372-4. DOI:10.1056/NEJMc2010419

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.