Approach to
Ischemic Stroke
Meaghan Wunder1
Published online: September 4 2021
1Schulich School of Medicine and Dentistry, Western University, ON, Canada
Corresponding Author: Meaghan Wunder, email mwunder@uwo.ca
DOI: 10.26443/mjm.v20i2.855
Abstract
An approach to managing acute ischemic stroke includes recognition, investigations, treatment, and secondary prevention. Firstly, facial drooping, limb weakness and slurred speech are some common signs that should raise the suspicion for stroke. Upon presentation, investigations, including the national institute of health stroke scale and a CT head, should be done to rule out intracranial hemorrhage and diagnose an ischemic stroke. The treatment principles for an acute ischemic stroke focus on removing or dissolving the occlusion to maintain or reinstate perfusion of the brain. Finally, patients suffering ischemic stroke should be admitted to the acute stroke unit and monitored for complications. Basic medical management of comorbidities should also be considered to prevent subsequent ischemic episodes. This article will explain each of these processes in more detail to help develop a basic approach to the management of an acute ischemic stroke.
Tags: Ischemic stroke, Infarction, Neurology
Question
Mr. Smith is a 72-year-old right-handed man who presents to the emergency room with sudden-onset right-sided weakness and numbness, as well as impaired speech. His partner drove him to the hospital when they noticed his smile was asymmetrical (right-sided facial weakness) and he could not lift his right arm. His past medical history includes type 2 diabetes and hypertension. His medications include metformin 850 mg, ASA 81 mg, atorvastatin 40 mg and lisinopril 10 mg. Vital signs are evaluated; heart rate: 123 beats per minute (bpm), respiration rate: 22 breaths per minute, blood pressure 172/91 mmHg, temperature 37.9 C. Physical exam reveals diffuse right arm weakness (MRC grade 2), and the right biceps and brachioradialis deep tendon reflexes are graded as 3+. Right plantar response is extensor. Language assessment reveals deficits in fluency, comprehension, repetition, and naming.
Investigations
EKG: rapid atrial fibrillation at 118 bpm
CBC and basic metabolic panel: WBC 7.8 x 109/L, Hgb 130 g/L, platelets 250 x 109/L, Na 140 mmol/L, Cl 109 mmol/L, HCO3 25 mmol/L, Creatinine 58 umol/L, urea 15 mmol/L, random blood glucose 8.4 mmol/L , INR 1.1
NIHSS evaluation: pending
Occlusion of which blood vessel would elicit the neurological symptoms presented above?
- left anterior cerebral artery
- basilar artery
- right middle cerebral artery
- left middle cerebral artery
- left posterior cerebral artery
Answer
D. Mr. Smith presented with acute onset right-sided weakness and numbness in his arm and face, as well as brisk right-sided upper limb reflexes on physical exam. These signs and symptoms are suggestive of a left hemisphere upper motor neuron lesion. Furthermore, his language deficits are indicative of a global aphasia with both expressive (i.e. producing language) and receptive (i.e. understanding language) components, localizing to the language areas of the left frontal (Broca’s area) and temporal-parietal (Wernicke’s area) lobes. The presence of cortical signs (aphasia), right upper motor neuron signs (weakness and hyperreflexia), and right-sided sensory symptoms (numbness) makes the most probable localization the left cerebral cortex, supplied by the left middle cerebral artery, making it the most likely vessel to be occluded. Anterior cerebral artery occlusions more often present with contralateral lower limb weakness and numbness. A basilar artery occlusion can result in “locked-in” syndrome, in which an individual is aware, but unable to communicate or move due to paralysis of most voluntary muscles in the body. Right middle cerebral artery occlusions present with left-sided sensory and motor symptoms, as well as neglect syndromes. Finally, a left posterior cerebral artery occlusion would more likely manifest as right-sided homonymous hemianopsia.
Initial Approach
Stroke is a time-sensitive diagnosis potentially resulting in a myriad of long-term functional deficits. It is a leading cause of disability in Canada, affecting over 400,000 individuals. (1) In acute ischemic stroke, “time is brain”, as treatment options and prognosis are both time-sensitive. In fact, complete occlusion of blood flow may lead to the death of vulnerable neurons after only 5 minutes. (2) It is therefore imperative that individuals have a practical approach to ischemic stroke, from recognition to recovery. The following article will describe such an approach at a level appropriate for those with basic clinical knowledge.
Basic management consists of timely recognition, diagnosis, and treatment, including 1) acute intervention (thrombolytic therapy and/or mechanical thrombectomy), 2) medical management in an acute stroke unit, and 3) secondary prevention measures and long-term follow-up. Each of these components will be briefly reviewed in the following sections.
Recognition
Though this paper will focus primarily on medical intervention, it is important to acknowledge the role of public health interventions for greater awareness and timely recognition. Such interventions depend on the participation of the public and medical professionals to succeed. For example, public health messaging such as the “FAST signs of stroke” campaign launched by the Heart and Stroke Foundation have been employed to hasten treatment and improve health outcomes. (4) The F.A.S.T. acronym stands for: Face – is it drooping? Arms – can you raise both? Speech – is it slurred or jumbled? Time – time to call 911 right away. (4) Indeed, in a time series evaluation of the FAST campaign in England, authors noted a significant positive impact on information-seeking behaviour and emergency admissions. (5)
Diagnosis
Initial evaluation of a suspected acute ischemic stroke consists of both good history taking, including time of onset and baseline functional status, and a focused neurological examination, often using the National Institutes of Health Stroke Scale (NIHSS). The NIHSS is a quantitative measure of stroke-related neurological deficit and is used to predict stroke severity and long-term outcome. (6) The scale evaluates level of consciousness, gaze, visual fields, facial palsy, motor strength, ataxia, sensation, language, dysarthria, and extinction/inattention in individuals showing signs of ischemic stroke. Upon confirmed suspicion of ischemic stroke, a non-contrast computed tomography (CT) scan is then ordered to rule out intra-cranial hemorrhage (ICH). A confirmed suspicion (from history and neurologic exam) along with a non-contrast CT with no evidence of ICH and no other explanation for the neurological deficits is sufficient to diagnose an acute ischemic stroke. Additionally, patients presenting with clinical suspicion for middle cerebral artery (MCA) stroke should be assigned an Alberta Stroke Program Early CT Score (ASPECTS) to quantitatively evaluate the degree of ischemic change on CT. (7) The score subtracts a point from a total score of 10 for each defined cortical region found to have evidence of early ischemic change. A score >8 suggests that patients may have a better chance for independence post stroke. (7)
Thrombolytic therapy
After the diagnosis of an acute ischemic stroke has been made, patients eligible to receive a tissue plasminogen activator (TPA) such as alteplase should receive treatment as soon as possible. (3, 8, 9) Intravenous (IV) alteplase should be dosed at 0.9mg/kg (not to exceed 90mg total dose), 10% given as a bolus and the remainder infused over 60 minutes. IV alteplase is administered to patients who have a blood pressure of less than 185/110 mmHg and do not fulfill any of the contraindications listed in Table 1, within 4.5 hours of stroke onset. (3) If blood pressure exceeds 185/110 mmHg, agents such as labetalol or nicardipine could be considered prior to TPA therapy. Importantly, obtaining blood work including troponins and INR should not delay administration of TPA, as long as there is no reason to suspect a coagulopathy. (3, 9) Also note that TPA should be administered even if mechanical thrombectomy is being considered. (3)
INR, international normalized ratio; aPTT, activated partial thromboplastin time; PT, prothrombin time; ASA, acetylsalicylic acid
Mechanical Thrombectomy
A CT angiogram should be performed at the same time as the diagnostic non-contrast CT to help determine eligibility for mechanical thrombectomy. Indications for mechanical thrombectomy include: a large vessel occlusion of the internal carotid or proximal middle cerebral arteries, less than 6 hours since symptom onset (2), age over 18, NIHSS score greater than 6, and no baseline neurological disability. (10) If patients present between 6 and 24 hours of symptom onset with anterior circulation occlusion, they may still be eligible for mechanical thrombectomy based on the result of a CT perfusion, diffusion-weighted magnetic resonance image (MRI), or MR perfusion scan. (10, 11) These studies measure blood flow to areas of the brain to help identify regions that have not yet been irreversibly damaged (the penumbra) and may benefit from reperfusion. (12) The mismatch ratio (MMR), which refers to the volume difference between the penumbra and the ischemic core, may be applied to predict response to reperfusion therapy; MMR >1.2 may predict greater response. (13)
Medical Management
Patients should then be admitted to the acute stroke unit, where the focus is on avoiding complications. During this time, blood pressure should be monitored and kept under 180/105 mmHg in those who received thrombolytic therapy to minimize the risk of a hemorrhagic event. Those ineligible for thrombolytic therapy should not undergo intervention for elevated blood pressure unless exceeding 210/120 mmHg. (14) Furthermore, all patients should be administered dual anti-platelet therapy within 24-48 hours (therapy should be held 24 hours in those who received TPA) to prevent recurrent thrombosis and ischemia. Brain imaging should also be repeated at 24 hours to assess the risk of hemorrhage. (3) Patients should also be kept nil per os (NPO) until assessed for dysphagia by a certified clinician or speech language pathologist. (3) Bed rest for only the first 12 hours after admission is recommended, as recent findings suggest that 12 (as opposed to 24) hours of bedrest is associated with significant reductions in pneumonia rates, discharge NIHSS scores, and length of stay. (15)
Secondary Prevention
As previously mentioned, all patients should be started on dual antiplatelet therapy and/or anticoagulation. (3) Patients who have suffered mild to moderate ischemic stroke who receive a combination of aspirin and clopidogrel have a lower risk of future major ischemic events over 90 days than those treated with aspirin alone. (16) As most recurrent ischemic events occur within the first week, dual antiplatelet therapy is recommended to be started within 24-48 hours and continued for at least 21-30 days post-ischemic stroke. (12) After this time, clopidogrel may be removed and while aspirin is continued indefinitely. (3) Anticoagulation should be reserved for those with identified atrial fibrillation or cardioembolic risk factors. Finally, secondary prevention should be addressed through the management of hypertension, dyslipidemia, and hyper/hypoglycemia during admission. Patients should also be given education regarding smoking cessation, physical activity, and nutrition before discharge. (3)
Beyond the Initial Approach
This section will discuss various mechanisms of ischemic stroke including embolism, decreased perfusion, and thrombosis. Understanding the major mechanisms by which ischemic stroke develops may guide secondary prevention through the reduction of pertinent risk factors.
Embolism
Embolism to the brain may affect both large and small cerebral vessels, leading to an acute ischemic stroke. The source of an embolus to the brain may be cardiac or arterial in origin, with left ventricular thrombi being an especially common source. Atrial fibrillation is an independent risk factor for cardioembolic stroke, regardless of its duration. Those with suspected cardioembolic stroke should therefore receive an echocardiogram for investigation of atrial fibrillation and subsequently be treated with oral anticoagulation to an optimal INR of 2.0 to 3.0. (17, 18) Note that anticoagulation in a patient with atrial fibrillation and acute cardioembolic stroke carries a risk of hemorrhage. For this reason, anticoagulation should be delayed several days in those with low risk of recurrence. In those with high risk of recurrence, especially if the infarct is not large and the patient does not have uncontrolled hypertension, early anticoagulation is still recommended. (17)
Perfusion Deficit
Acute ischemic stroke may also be due to stenosis of large and small cerebral vessels leading to perfusion deficits, with atherosclerotic plaques being a major factor in the development of vessel stenosis and occlusion. Regarding large artery atherosclerotic plaques, stenoses resulting in occlusion of more than 70% identified with angiography predispose individuals for ischemic events, including thrombo-embolic complications. Medical and surgical interventions available for these patients include anticoagulation (as described above) and carotid endarterectomy, respectively. Hypoperfusion of small (lacunar) arteries is another important source of acute ischemic stroke. Lacunar strokes are due to occlusion of small, deep, perforating arteries in cerebral circulation and often present with isolated motor or sensory deficits. Etiology may be due to cardiac or arterial embolism, plaque embolism, or even large vessel stenosis. In fact, lacunar hypoperfusion may be a first indication of large vessel stenosis. Measures to reduce atherosclerotic risk factors are therefore beneficial for secondary and primary prevention of vessel stenosis that causes acute ischemic stroke. (17) Lifestyle modifications including regular exercise, smoking cessation, and adequate nutrition are particularly pertinent in preventing atherosclerotic plaques that arise from hypertension, dyslipidemia, and hyperglycemia. (18)
Thrombosis
Thrombosis (and prothrombotic states) is another important mechanism of acute ischemic stroke. Thrombosis may be secondary to atherosclerotic plaque rupture, or primary due to hematological abnormality. In the case of atherosclerotic plaque rupture, medical management of the atherosclerotic risk factors described above may be helpful in secondary prevention. Primary prothrombotic states due to hemostatic abnormalities in antithrombins, heparin cofactor II, proteins C and S and fibrinolytic factors may be associated with stroke at any age, and in these patients long-term anticoagulation with warfarin is generally recommended. (17, 18)
Conclusion
In acute ischemic stroke, quick and efficient recognition and activation of the multidisciplinary team are critical for timely treatment. Upon emergency room arrival, stroke severity should be estimated based on the NIHSS, and hemorrhagic stroke should be ruled out based on non-contrast CT. After the diagnosis of acute ischemic stroke, TPA should be administered within the first 4.5 hours. If the stroke is identified as a large vessel occlusion, mechanical thrombectomy should be considered within the first 6-24 hours. Finally, stroke patients should be started on dual anti-platelet therapy and admitted to the hospital for additional investigations and management. Elucidation of the stroke etiology may aid in guiding additional investigations and secondary prevention.
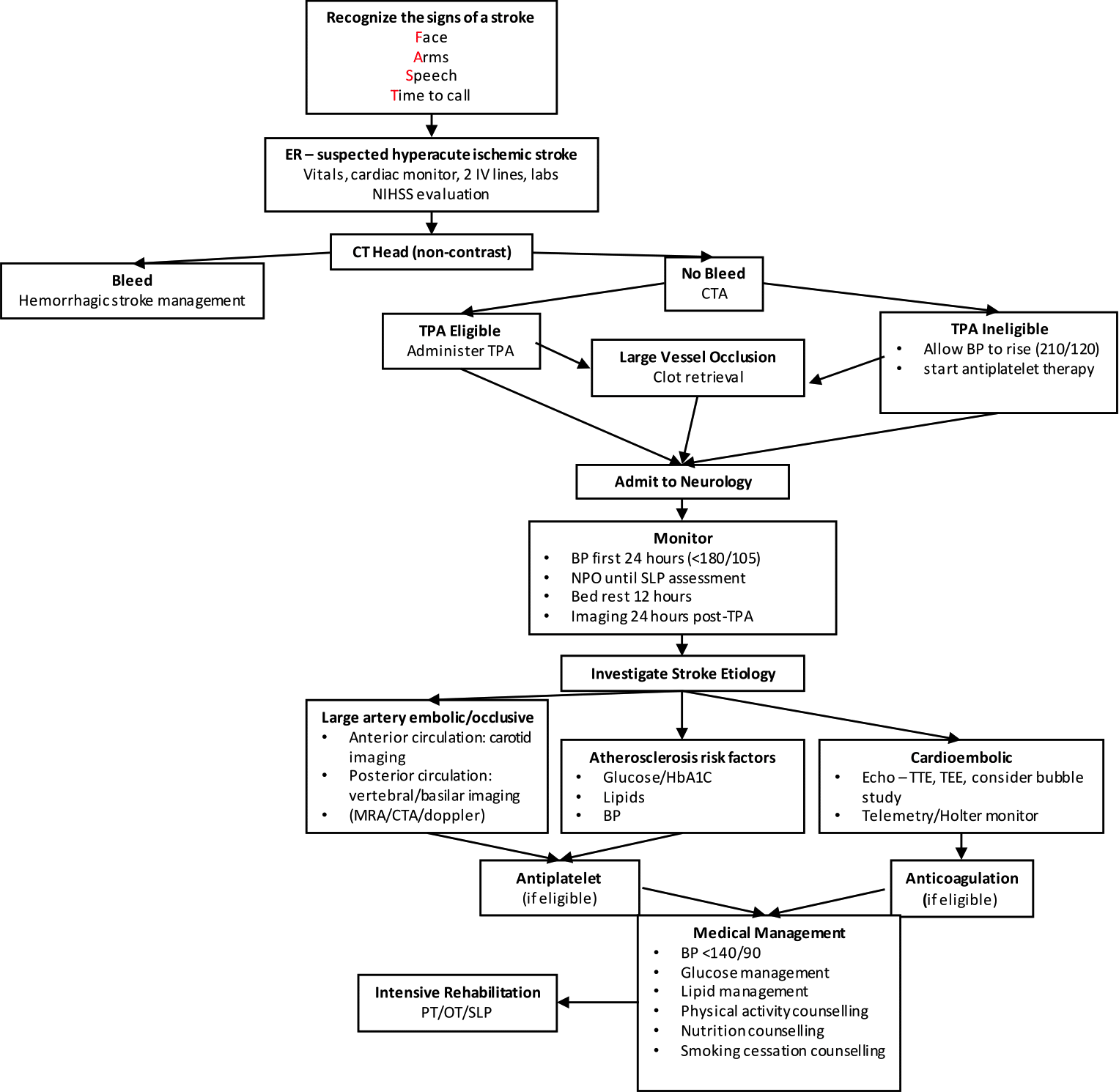
ER, emergency room; NIHSS, national institute of health stroke scale; CT, computed tomography; CTA, computed tomography angiography; TPA, tissue plasminogen activator; BP, blood pressure; NPO, non-per-oral; MRA, magnetic resonance angiography; TTE, transthoracic echocardiogram; TEE, transesophageal echocardiogram; PT, physical therapy; OT, occupational therapy; SLP, speech-language pathologist.
References
- Krueger H, Koot J, Hall RE, O’Callaghan C, Bayley M, Corbett D. Prevalence of Individuals Experiencing the Effects of Stroke in Canada: Trends and Projections. Stroke. 2015 Aug;46(8):2226–31.
- Lee J-M, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000 Sep 15;106(6):723–31.
- Powers William J., Rabinstein Alejandro A., Ackerson Teri, Adeoye Opeolu M., Bambakidis Nicholas C., Becker Kyra, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec 1;50(12):e344–418. https://doi.org/10.1161/STR.0000000000000211
- FAST Signs of Stroke...are there other signs? [Internet]. Heart and Stroke Foundation of Canada. [cited 2021 Feb 9]. Available from: https://www.heartandstroke.ca/en/stroke/signs-of-stroke/existe-t-il-d-autres-signes-de-l-avc-que-vite/
- Flynn D, Ford GA, Rodgers H, Price C, Steen N, Thomson RG. A time series evaluation of the FAST National Stroke Awareness Campaign in England. PloS one. 2014;9(8):e104289–e104289. https://doi.org/10.1371/journal.pone.0104289
- Schlegel Daniel, Kolb Stephen J., Luciano Jean M., Tovar Jennifer M., Cucchiara Brett L., Liebeskind David S., et al. Utility of the NIH Stroke Scale as a Predictor of Hospital Disposition. Stroke. 2003 Jan 1;34(1):134–7.
- Mokin M, Primiani CT, Siddiqui AH, Turk AS. ASPECTS (Alberta Stroke Program Early CT Score) Measurement Using Hounsfield Unit Values When Selecting Patients for Stroke Thrombectomy. Stroke. 2017 Jun;48(6):1574–9.
- Boulanger JM, Lindsay MP, Gubitz G, Smith EE, Stotts G, Foley N, et al. Canadian Stroke Best Practice Recommendations for Acute Stroke Management: Prehospital, Emergency Department, and Acute Inpatient Stroke Care, 6th Edition, Update 2018. International journal of stroke. 2018;13(9):949–84. https://doi.org/10.1177/1747493018786616
- Powers WJ. Acute Ischemic Stroke. The New England Journal of Medicine. 2020;383(3):252–60. https://doi.org/10.1056/NEJMcp1917030
- Mokin M, Ansari SA, McTaggart RA, Bulsara KR, Goyal M, Chen M, et al. Indications for thrombectomy in acute ischemic stroke from emergent large vessel occlusion (ELVO): report of the SNIS Standards and Guidelines Committee. Journal of NeuroInterventional Surgery. 2019 Mar 1;11(3):215–20.
- Chugh C. Acute Ischemic Stroke: Management Approach. Indian J Crit Care Med. 2019 Jun;23(Suppl 2):S140–6.
- Phipps MS, Cronin CA. Management of acute ischemic stroke. BMJ. 2020 Feb 13;368:l6983.
- Demeestere J, Wouters A, Christensen S, Lemmens R, Lansberg MG. Review of Perfusion Imaging in Acute Ischemic Stroke. Stroke. 2020 Mar 1;51(3):1017–24.
- Aiyagari Venkatesh, Gorelick Philip B. Management of Blood Pressure for Acute and Recurrent Stroke. Stroke. 2009 Jun 1;40(6):2251–6.
- Silver B, Hamid T, Napoli MD, Behrouz R, Khan M, Saposnik G, et al. Twelve versus twenty four hour bed rest after acute ischemic stroke reperfusion therapy (P5.204). Neurology [Internet]. 2018 Apr 10 [cited 2021 Apr 23];90(15 Supplement). Available from: https://n.neurology.org/content/90/15_Supplement/P5.204
- Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. New England Journal of Medicine. 2018 Jul 19;379(3):215–25. https://doi.org/10.1056/NEJMoa1800410
- Hankey GJ. Secondary stroke prevention. The Lancet Neurology. 2014 Feb 1;13(2):178–94.
- Panel, Mohr J. P., Albers Gregory W., Amarenco Pierre, Babikian Viken L., Biller José, et al. Etiology of Stroke. Stroke. 1997 Jul 1;28(7):1501–6.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.