Approach to
Venous Thromnoembolism
Salima Ramdani1
Published online: October 13, 2021
1McGill University
Corresponding Author: Salima Ramdani, email salima.ramdani@mail.mcgill.ca
DOI: 10.26443/mjm.v21i1.851
Abstract
Venous thromboembolisms can manifest as a spectrum of diseases and complications, such as deep vein thrombosis (DVT) and pulmonary embolism (PE), as a consequence of hypercoagulability, endothelial damage and/or venous stasis. DVT can present as localized pain or heaviness, unilateral edema, dilatation of superficial nonvaricose veins, a palpable cord or Homans’s sign. Symptoms of PE include acute or worsening shortness of breath and pleuritic chest pain while physical examination may be remarkable for tachycardia and tachypnea. However, given their non-specificity, using these signs and symptoms alone allows for poor differentiation between VTE and other entities. This review will focus on a multi-step diagnostic tree allowing for evidence-based interpretation of tests following a determined pre-test probability (PTP), as per Thrombosis Canada recommendations and ASH clinical guidelines. An introduction to VTE in Pediatrics and pregnancy will also be discussed.
Tags: Thrombosis,venous thromboembolisms, pulmonary embolism, DVT, PE, Virchow’s triad.
Case and Question
A 75-year-old man presents to the emergency room with a one-day history of new-onset dyspnea and right calf pain and swelling. Review of systems was notably negative for chest pain, palpitation, and fever. He is known for metastatic small cell lung carcinoma, hypertension and dyslipidemia. His medications include codeine, amlodipine and atorvastatin, and his chemotherapy regimen includes cisplatin and etoposide. He denies any allergies. He is normotensive (142/80), tachycardic at 111 beats per minute, tachypneic (22 breaths/min) with good oxygen saturation (96% on room air). His physical examination is remarkable for unilateral swelling of the right lower extremity and a positive Homans’s sign. An ECG done in triage shows sinus tachycardia, and nonspecific T-wave inversions unchanged from previous ECGs.
You already have the following laboratory values:
- Hemoglobin: 100 g/L (normal: 130-170 g/L)
- Platelet count: 380 x 109/L (normal: 130-400 x 109/L)
- White blood cell count: 7 x 109/L (normal: 4-10 x 109/L)
- Sodium: 136 mmol/L (normal 136–146 mmol/L)
- Potassium: 4 mmol/L (normal 3.5–5.1 mmol/L)
- Chloride: 98 mmol/L (normal 98–100 mmol/L)
- Bicarbonate: 26 mmol/L (normal 21–32 mmol/L)
- Creatinine: 64 µmol/L (normal: 49–93 µmol/L)
What is the next best step?
- Order a Chest X-Ray (CXR), Troponins, D-dimer
- Order a D-dimer and Whole-leg ultrasound
- Order a CT pulmonary angiogram (CTPA) and start anticoagulation
- Order an echocardiogram then a V/Q lung scan
Answer
C. Order a CTPA and start anticoagulation.
This patient has a history of malignancy, received chemotherapy within the last 6 months, had clinical signs and symptoms of deep vein thrombosis (pain, swelling, erythema, positive Homans’s sign), he is tachycardic and pulmonary embolism is the most likely diagnosis (PE) (1). Given his presentation, this patient’s Wells score is above 4.5 which puts him in the likely PE risk category (1). A high-sensitivity D-dimer is most useful to rule out PE when it is unlikely (1). Certain centres may also choose to order a CXR and troponins to investigate other differential diagnoses, however the most important given his high-risk is to undergo a CTPA to confirm the diagnosis of PE. A V/Q lung scan can be considered in patients with renal dysfunction or contrast allergy for example; however, this study is best suited for patients that do not have intraparenchymal lung pathologies (1). An echocardiogram can be helpful especially when a patient is too unstable to undergo CTPA, but does not provide a definitive diagnosis of PE (1). Anticoagulation should be started especially if this study cannot be done within 4 hours and if there are no contraindications (2).
Initial Approach
With an annual incidence of 45,000 Canadians, venous thromboembolisms (VTEs) can manifest as a spectrum of diseases and complications, such as deep vein thrombosis (DVT) and pulmonary embolism (PE), as a consequence of hypercoagulability, endothelial damage and/or venous stasis (3, 4). Mirroring Virchow’s triad, the risk factors of VTEs include prolonged immobilization, hormonal therapy, surgery, trauma, as well as malignancy, pregnancy, myeloproliferative disorders and inherited thrombophilia (3). Nonetheless, 50% of PEs presenting for the first time are idiopathic (1).
DVTs can present as localized pain, heaviness, swelling and/or discoloration of the affected extremity. Signs may vary from unilateral edema (with a difference ≥2cm in the circumference of the limb), dilatation of superficial nonvaricose veins, a palpable cord or a positive Homans’s sign (tenderness with dorsiflexion of the foot) (3, 5). Upper extremity DVT, which may arise in the context of venous catheter, Paget-Schroetter syndrome and other conditions, is however less common (4). Although only 25% to 50% of PEs present with clinical evidence of DVT, PEs are commonly a complication of proximal DVTs (1). Symptoms of PE include acute or worsening shortness of breath, pleuritic chest pain, hemoptysis and/or syncope, while physical examination may be remarkable for tachycardia and tachypnea (6). In submassive or massive PEs, ECG may show a right bundle branch block, T-wave inversion of precordial leads or a S1Q3T3 Pattern, which are manifestations of right ventricular strain (6) However, the most common ECG finding remains sinus tachycardia (6). CXR is most useful in assessing for other potential diagnoses as the Hampton hump (wedge-shaped opacification) or Westermark sign (decreased vascularity distal to the PE), which are signs suggestive of PE, but that are rarely seen (6).
Given their non-specificity, using these signs and symptoms alone allows for poor differentiation between VTEs and other differential diagnoses, such as cellulitis and superficial thrombophlebitis for DVTs, or pericarditis and congestive heart failure for PEs (5). This review will focus on a multi-step diagnostic tree allowing for evidence-based interpretation of tests following a determined pre-test probability (PTP).
Deep Venous Thrombosis
1. Pre-Test Probability (PTP)
The Wells clinical prediction rule for DVT (Figure 1) is a validated model that stratifies patients based on their risk for DVT (7). Taking into account their risk factors and presentation, the score given will determine the risk category of the patient. For example, a score of 0 corresponds to a PTP of 5.0% (95% CI, 4.0%-8.0%) but a score higher than 3 corresponds to a PTP of 53% (95% CI, 44%-61%) of DVT (7, 8). A score of 0 or 1 implies that DVT is unlikely, whereas a score of 2 and above means that DVT is likely.
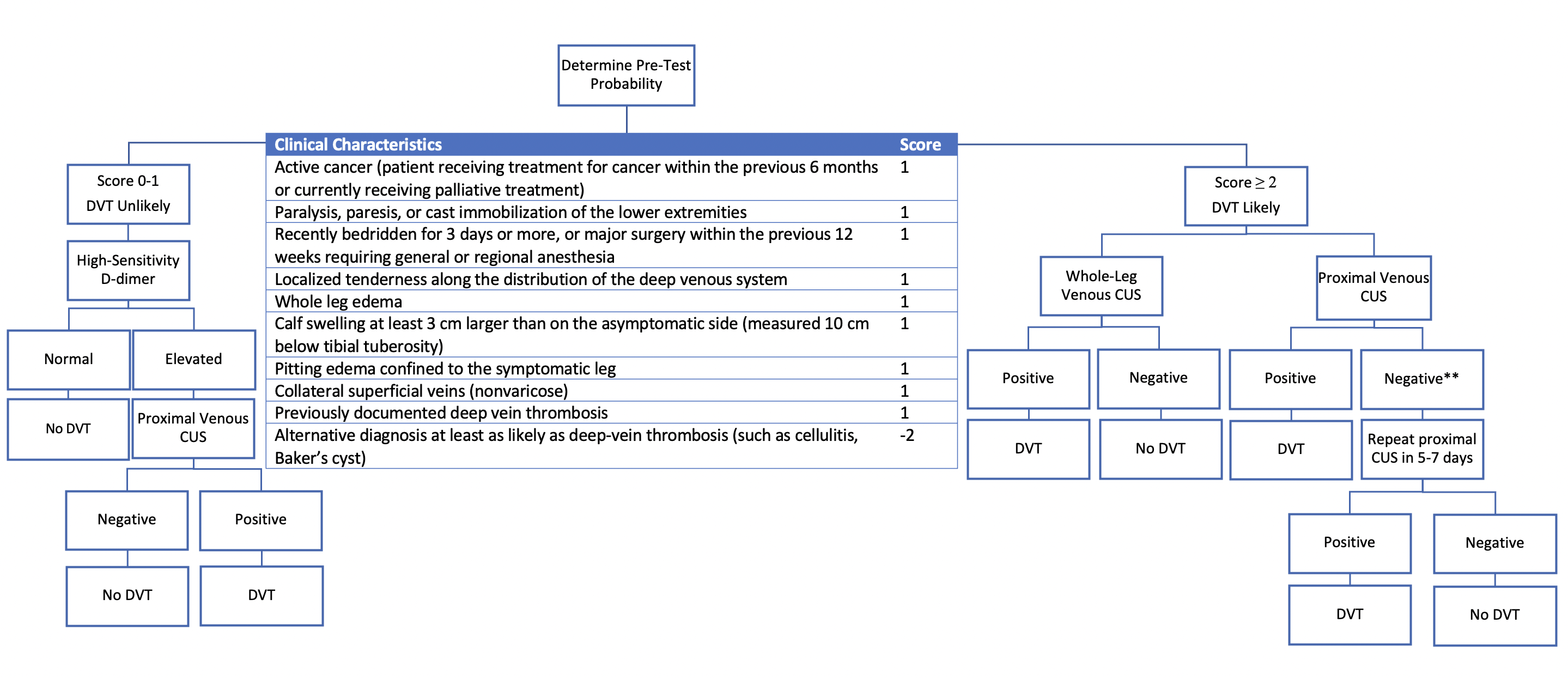
2. D-Dimer
OD-dimer assays measure the product of fibrin degradation. They are highly sensitive but poorly specific as they can be elevated in other inflammatory conditions, such as malignancy or following surgery (7). A high-sensitivity D-dimer assay is helpful in the unlikely DVT risk group to “rule out’’ a VTE if normal, while an elevated D-dimer would warrant further investigations (4, 8). In the likely DVT risk category, the D-dimer result, whether elevated or not, would not change your likelihood sufficiently to rule out DVT given its high initial PTP, which is why ultrasound imaging is directly the next step.
3. Venous Compression Ultrasound
Thrombosis Canada recommends proximal leg compression ultrasound (CUS) for evaluation of DVT, as non-compressibility of the deep veins below the deep fascia suggests a high likelihood of DVT (4). In patients with a high PTP, a repeat proximal CUS should be sought 5-7 days following a negative CUS, given that 20% of distal DVTs may extend proximally (4). Limitations of this study include distinguishing an acute clot from a chronic clot and operator variation (3). Whole-leg ultrasound (including distal veins) is used in some centres, in which case a repeat CUS is not warranted if negative (4, 9). Another less common imaging modality is CT Venography, which can be useful with proximal iliac DVTs or in May Thurner syndrome (3).
Pulmondary Embolism
Given certain patients may present with complications of PE, it is important to first assess the hemodynamic stability of the patient. If the patient is stable with a blood pressure >90/60 mmHg, starting with determining the pre-test probability is best. A moderate or large PE can lead to right heart failure and subsequent cardiogenic shock . In fact, in a patient with massive PE, CTPA may not be possible as they may be too unstable (3). In such scenarios, an urgent echocardiogram may give clues of massive PE with the presence of signs of right heart overload, presence of right ventricle or main pulmonary artery embolus and elevated pulmonary artery systolic pressure (1, 6). If so, treatment should be started, especially if there are no alternative diagnoses (1). The diagnosis should still be confirmed when the patient is able to tolerate CTPA (1, 9).
1. Pre-Test Probability (PTP)
The Wells clinical prediction rule for PE (Figure 2) is a validated model, initially used in the emergency population, that stratifies patients based on their risk of PE (10, 11). PE is unlikely with a score of 0-4, which corresponds to a PTP of 12.1% (95% CI, 10.7%-13.5%) (12). In the emergency room setting, meeting all criteria for the PE Rule-out Criteria (PERC) rules out PE without the need for D-dimer testing (1, 9, 12). PERC criteria are met if a patient is less than 50 years old, not tachycardic (heart rate less than 100 beats/min), not hypoxemic (with oxygen saturation higher than 94%), does not have a history of surgery or trauma in the last 4 weeks, a history of VTE or estrogen use, and does not present with unilateral leg swelling or hemoptysis (11, 12). A patient is likely to have PE with a Wells score of 4.5 and above, with PTP of 37.1% for that risk category (95% CI, 34.2%-40.0%) (12).
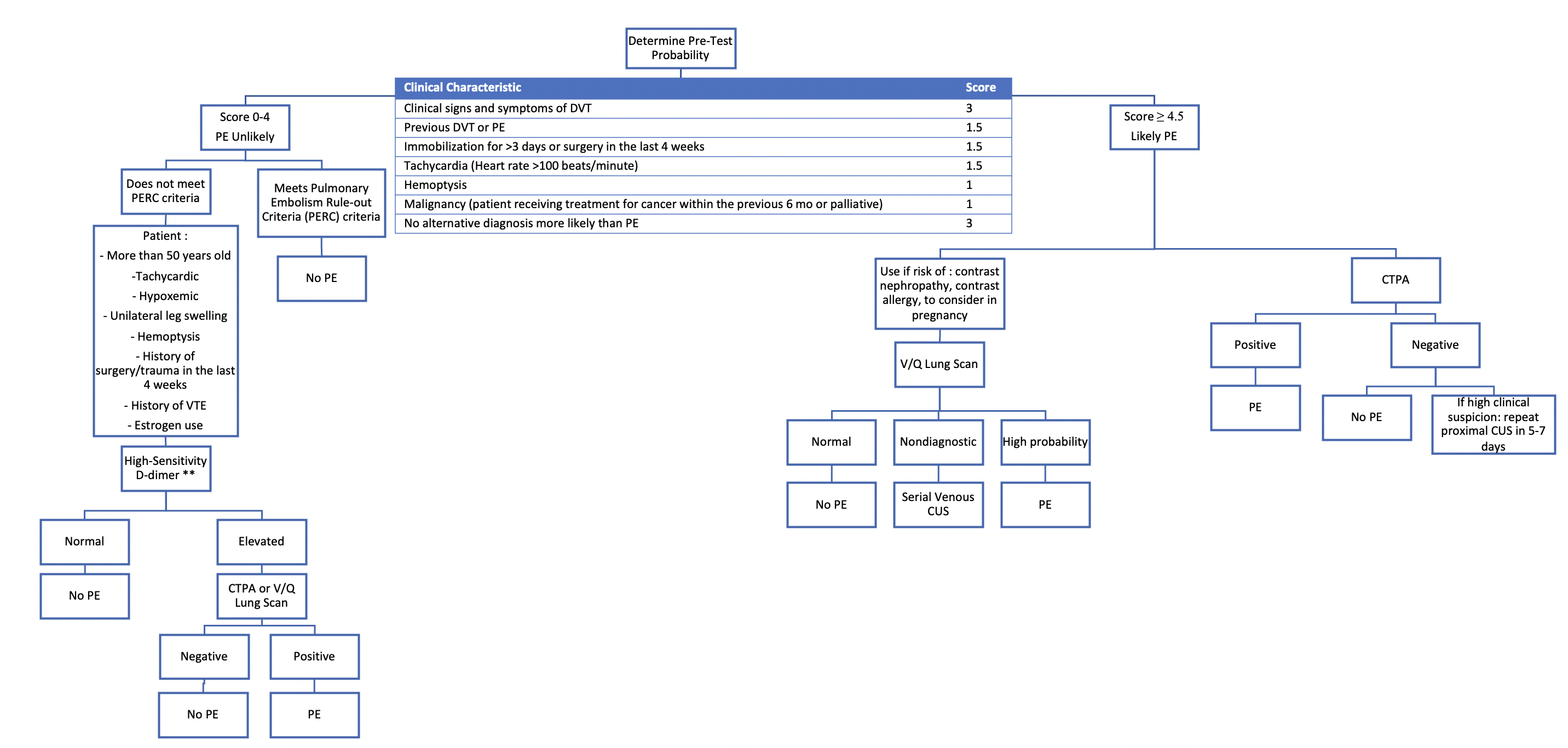
2. D-Dimer
Once again, high sensitivity D-dimer assays may be used in patients in the unlikely risk category as they rule out PE if normal given their high negative predictive value (12). Age-adjusted D-dimer cut-offs have been validated for PE such that the cut-off increases after 50-years-old by their age x 10ng/L. Its use is institution-dependent and was found to be as safe as standard cut-off when used in the outpatient setting (1, 9). If the high-sensitivity D-dimer is positive or elevated, further investigations are necessary (1).
3. Multidetector Computed Tomography Pulmonary Angiogram (CTPA/CTPE)
With its high sensitivity and specificity, multidetector CTPA is the next step in likely PE patients as well as the unlikely risk group with a positive high-sensitivity D-dimer (1). It has largely replaced pulmonary angiography given it is less invasive (3). Following intravenous contrast injection, PE is identified by pulmonary arteries filling defects (3). Follow-up with venous CUS or V/Q lung scan may be considered in patients with high clinical suspicion despite a negative CTPA and is recommended by the ASH guideline panel (1, 9).
4. Ventilation-Perfusion (V/Q) Lung Scan
Given the risk of contrast nephropathy, contrast allergy and radiation exposure associated with CTPA, a V/Q lung scan study may be chosen in certain patients and is recommended by the ASH guideline panel for the unlikely risk group (1, 9). Pulmonary arterial filling defects are identified by any mismatches in ventilation to perfusion following radioisotope administration (3). This study has a high sensitivity and specificity for patients without significant lung disease. A V/Q scan may be nondiagnostic in older patients and those with pre-existing lung pathologies (such as COPD), in which case serial venous CUS or even CTPA can be undertaken as a next step to aid in the diagnosis (9).
Treatment
As a first step, it is important to address any signs of instability patients may present, such as hypoxemia or hypotension. For high-risk patients with more than four hours of delay for their diagnosis, it is important to consider a rapidly acting anticoagulant while waiting for imaging or other tests, unless there are contraindications (such as active bleeding) (5). While most DVTs can be managed in an outpatient setting, PEs should be risk stratified. Two tools are available from Thrombosis Canada: The Pulmonary Embolism Severity Index [PESI], which has 5 classes of severity, and the Simplified PESI (2). Patients with low or very low-risk scores by the PESI can be managed as outpatients, while the following characteristics should generally prompt inpatient treatment instead: severe renal dysfunction, high bleeding risk, history of cancer or of cardiopulmonary disease, hypotension, hypoxemia, tachycardia or age above 80-years-old (2, 6).
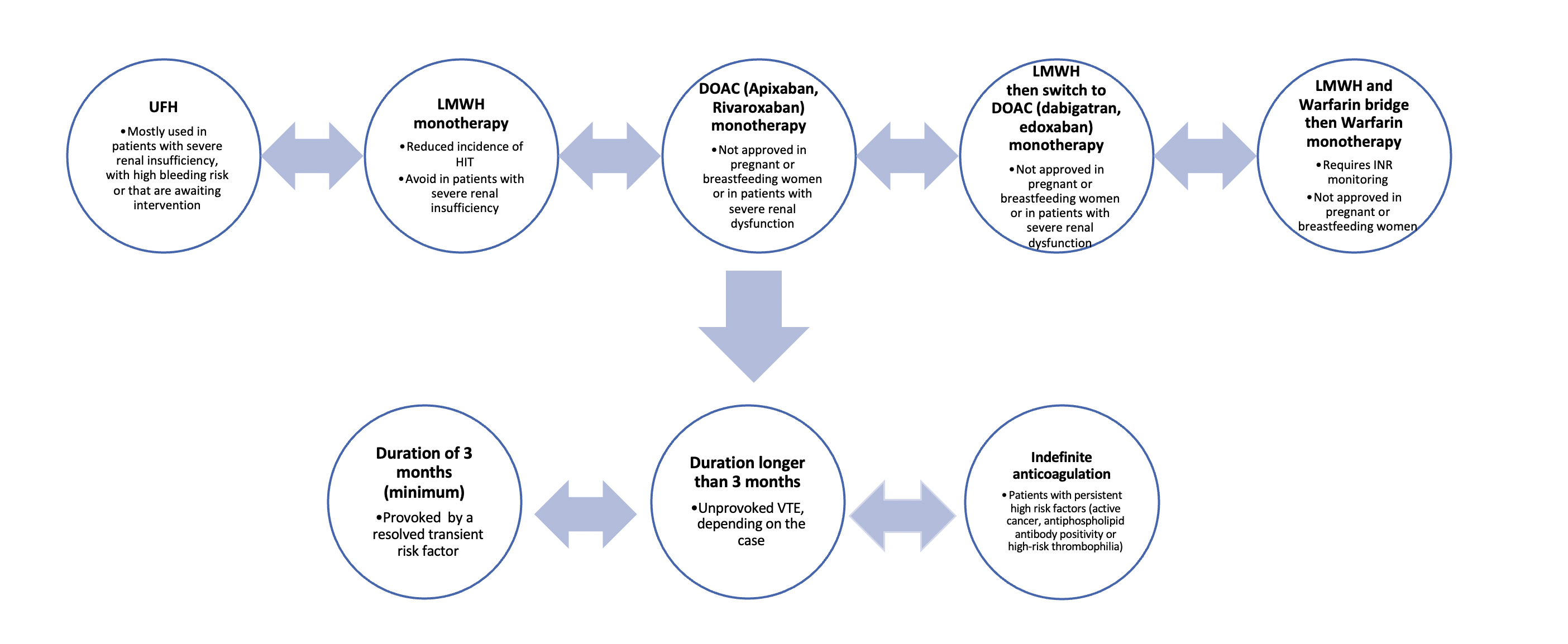
Once a diagnosis of VTE is established, there are 3 general principles of initial treatment (Figure 3). First, monotherapy is available by the administration of the direct-acting oral anticoagulants (DOACs) apixaban or rivaroxaban, unfractionated heparin (UFH) or low molecular weight heparin (LMWH) (2, 5). UFH is mostly useful in patients with severe renal failure and unstable patients who may require interventions (thrombolytic therapy or inferior vena cava filter placement) given the short half-life and easy reversibility, while the inhibitor of factor Xa fondaparinux can be used in heparin-induced thrombocytopenia for example. Second, the DOACs dabigatran and edoxaban have been shown to have lower bleeding risk in non-cancer patients, but treatment should start by LMWH for 5-10 days, before switching to these specific DOACs (2, 5). Finally, warfarin, a vitamin K antagonist, can be also used for the treatment of VTE but requires to be started with LMWH for at least 5 days, as the international normalized ratio (INR) should be above 2.0 for two consecutive days prior to warfarin monotherapy (13). The LMWH counteracts the initial prothrombotic effect of warfarin (3). A fourth treatment option, thrombolysis, is considered for massive PEs but has significant morbidity given its high risk of bleeding and hemorrhagic stroke and its discussion is beyond this review (9). Finally, if a patient with an acute proximal DVT or acute PE has any contraindications for anticoagulation therapy, the possibility of using a vena cava filter should be raised (2).
Duration of treatment is a balancing act between the risk of recurrence and of bleeding. This complication occurs mostly during the first 3 months of treatment and the highest risk factors are: cancer, thrombocytopenia, chronic kidney or liver disease and antiplatelet therapy (5, 14). As a general principle, patients with a VTE provoked by a transient resolved risk factor (such as surgery in the last 3 months, reduced mobility, hormonal therapy, pregnancy) can stop their anticoagulant treatment after a minimum of 3 months (9, 14). In patients with unprovoked VTE, anticoagulation therapy should be pursued for at least 3 months, and further therapy should be discussed (14). Patients with persistent strong risk factors, such as active cancer, antiphospholipid antibody positivity or high-risk thrombophilia, may require lifelong anticoagulation (14).
This section covers an introduction to the approach to VTE in special populations.
Pediatric VTEs often present secondary to a multitude of risk factors from surgery, central lines, inherited thrombophilic conditions and more (15). In fact, central-access vascular catheters are related to 90% of neonatal VTEs (15). Because clinical decision tools or markers such as the Wells score and D-dimer assays have not been validated in pediatrics, diagnosis of DVT can be sought by CUS and doppler while PE needs to be confirmed with CTPA or even magnetic resonance venography (MRV) (15). The use of DOACs is not yet approved in pediatrics, as such UFH, LMWH and warfarin are first-line for anticoagulation therapy, for a duration of at least 3 months if provoked, or longer depending on the clinical scenario (15).
The incidence of VTEs, commonly occurring from peripartum to 6-12 weeks postpartum, is about 1.5 per 1,000 pregnancies in Canada (16). Most present as isolated iliofemoral DVT of the left lower extremity, and it can be difficult to distinguish DVT or PE symptoms given dyspnea and leg swelling are common in pregnancy. The LEFt rule is a risk stratification strategy that can be used for DVTs where points are allocated when the patient presents during the first trimester, with left leg symptoms and/or edema of the leg (with a 2 cm difference). However, venous CUS remains the first diagnostic choice (16, 17). For PE diagnosis, although CTPA has less risk of radiation to the foetus (below 50mGy for both CTPA and V/Q scan), there is higher concern for breast irradiation cancer risk (16). Of note, V/Q lung scan can affect breastmilk up to 48 hours after the study (16). The Pregnancy-Adapted YEARS Algorithm can be used according to Thrombosis Canada (16). Because warfarin can cross the placenta and DOACs have not been approved for use in pregnancy, and the first-choice therapy stays LMWH or UFH (2, 13).
Although the initial diagnostic tree will be similar, it is important to recognize when to do thrombophilia testing, such as in patients with a strong family history of VTE, recurrent VTEs or spontaneous VTE in young patients or at unusual sites unexplained by other causes (4, 15). The most common, heterozygosity for factor V Leiden or prothrombin G20210A mutations, are considered low-risk thrombophilia and generally do not require longer anticoagulation therapy (14, 15). Deficiency for antithrombin, protein C or protein S is however more potent and may require lifelong anticoagulation (14, 15).
Beyond Initial Approach
1. Pediatrics
2. Pregnancy
3. Thrombophilias
References

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.