Approach to
Heart Failure
Delphine Hansen1
Published online: 19 March 2021
1McGill University, Montreal, QC, Canada
Corresponding Author: Delphine Hansen, email delphine.hansen-jaumard@mail.mcgill.ca
DOI: 10.26443/mjm.v19i1.518
Abstract
Heart Failure (HF) affects more than 650,000 Canadians (3.6% of Canadian adults above age 40). Even with recent advances in the diagnosis and treatment of this disease, HF remains among the five most common causes for hospitalization in Canada, with a readmission rate above 30% at the 1-year mark. Despite the significant economic and clinical burden of this disease, there is limited awareness among healthcare providers, healthcare system managers, and governments regarding the current HF epidemic and available therapies. This article reviews the definition of HF and the approach to evaluating a patient with suspected HF, focusing on the different presentations of HF, the clinical significance of ejection fraction, and the usefulness of BNP as a marker of cardiac function.
Tags: Heart Failure, Ejection Fraction, Dyspnea, Pulmonary Edema, Brain Natriuretic Peptide (BNP)
Question
A 61-year-old male presents to the Emergency Department with a 4-month history of progressive exertional dyspnea and fatigue. He reports frequently waking up due to a feeling of breathlessness. He complains of a 10-pound weight gain in the past month. Past medical history is significant for hypertension, coronary artery disease (CAD), dyslipidemia, and obesity—for which he takes atorvastatin and perindopril. He has a 40 pack-year history of cigarette smoking. On cardiopulmonary auscultation, there are diffuse bilateral crackles in the lower third fields of the lungs and a third heart sound (ventricular gallop). Vital signs are: pulse of 118, respiratory rate of 26, blood pressure of 164/93, O2 saturation 92%, and body temperature of 36.9°C. On physical exam, you note mildly laboured breathing, a distended jugular vein, and bilateral edema of the lower extremities.
Which of the following statements is true regarding this patient?
- This patient should be started on a short-acting bronchodilator, a long-acting bronchodilator, and a corticosteroid to improve respiratory status.
- Measurement of brain natriuretic peptide (BNP) is the gold-standard diagnostic tool for this patient’s condition.
- If the heart echocardiogram shows a left ventricular ejection fraction (LVEF) < 40%, a triple therapy consisting of an ACE inhibitor (ACEi), a beta-blocker (BB) and a mineralocorticoid receptor antagonist (MRA) should be started to decrease this patient’s risk of mortality.
- If the heart echocardiogram shows a LVEF > 50%, the likelihood of a diagnosis of heart failure is low.
Answer
C. This patient’s clinical presentation is highly suggestive of heart failure (HF). The three classes of medication proven to reduce HF mortality with reduced LVEF are ACEi, BB, and MRA, thus constituting the basic initial triple therapy for patients with this condition. (1)
A. False. This pharmacotherapy would be indicated for a patient with chronic obstructive pulmonary disease (COPD). Although both COPD and HF can present with dyspnea, dyspnea in HF is more likely due to pulmonary edema and should be treated with diuretics, not inhalers.
B. False. HF is a clinical diagnosis that should be confirmed with an echocardiogram.
E. False. HF can present with a preserved ejection fraction.
Overview of Heart Failure
What is Heart Failure?
Heart failure is a clinical syndrome characterized by signs and symptoms of reduced cardiac output and/or pulmonary and systemic volume overload. (2,3) Signs and symptoms include dyspnea, orthopnea (dyspnea in an inclined or supine position), paroxysmal nocturnal dyspnea (dyspnea most severe at night, often leading to nighttime awakening), fatigue, weakness, exercise intolerance, dependent edema, cough (particularly in the decubitus position), weight gain (due to water retention), abdominal distension, nocturia, and cool extremities. (3)
Etiologies and Risk Factors
HF often results from a previous insult or disease process in the heart. The most common causes include CAD, hypertension, idiopathic cardiomyopathy, and valvular heart disease. Less frequent etiologies include arrhythmias, connective tissue diseases such as lupus, endocrine and metabolic disorders such as diabetes or thyroid disease, myocarditis, and pericarditis. (2) Other risk factors for HF include heavy alcohol or substance use, smoking, physical inactivity, obesity, diabetes, chemotherapy or radiotherapy, and lower socioeconomic status. (1,2)
Epidemiology
HF affects more than 650,000 Canadians (3.6% of Canadian adults aged 40 and above). Prevention, diagnosis, and treatment of HF are paramount because the all-cause mortality for Canadians living with this disease is four to six times higher than those living without the condition. (4)
Initial Approach
Evaluating a patient with suspected HF begins with a thorough medical history and physical examination. It is important to remember that symptoms suggestive of HF include exertional dyspnea, intolerance to exercise, edema, fatigue, orthopnea, paroxysmal dyspnea, and weight gain. Signs suggestive of HF include elevated jugular venous pressure, large abdomen (may include ascites and hepatojugular reflex), cool extremities, dependent edema, and labored breathing (with or without rales). (1-5)
On cardiac auscultation, both bradycardia and tachycardia can be found, as well as a third heart sound (ventricular gallop). (1,5) Finally, in a patient with a clinical presentation suggestive of HF, attention should be given to signs and symptoms suggestive of a particular HF etiology: chest pain or recent myocardial infarction (CAD), skin, joint or eye lesions (sarcoidosis), recent fevers, viral infection, history of IV drug use (endocarditis, myocarditis, pericarditis), syncope (bradycardia, heart block, or other arrhythmias). (2,5)
Initial investigations in suspected HF are aimed at gathering evidence suggestive of the diagnosis, ruling out other possible causes for the symptoms, determining potential etiologies, and identifying complications and comorbid illnesses (see Table 1).
| Table 1: Suggested initial investigations for suspicion of heart failure | Investigation | Rationale |
| Chest X-Ray | Rule out pulmonary disease (e.g. pneumonia, pulmonary mass) Assess for pulmonary edema, cardiomegaly and pleural effusions |
| Electrocardiogram (ECG) | Rule out arrhythmias, acute or previous MI Determine if patient needs concurrent referral to cardiology (for example, presence of a heart block necessitating a pacemaker) |
| Complete blood count (CBC) | Rule out concomitant anemia or an infectious process |
| Electrolytes, including magnesium, phosphate, and calcium | Rule out electrolyte imbalances which can cause arrhythmias (particularly if the patient is on diuretics) |
| Renal function | Rule out renal causes of volume overload Assess renal function (may influence the choice of treatment) |
| Urinalysis | Rule out renal causes or infection |
| Glucose | Rule out hyperosmolar state |
| Thyroid function | Rule out concomitant thyroid disorder |
| Brain natriuretic peptide (BNP) | Rules out HF if low Can raise suspicion for HF if high Can be used as a baseline value for monitoring purposes during treatment |
There is no gold standard diagnostic test for HF. One of the most widely accepted diagnostic aid scores is the Framingham Diagnostic Criteria for Heart Failure (available at: https://www.mdcalc.com/framingham-heart-failure-diagnostic-criteria). (2,6) Even though many scores exist to determine the likelihood of HF, it remains a clinical diagnosis where one must consider clinical presentation, scores, and investigations (See Flow chart 1). (2,3,7,8)
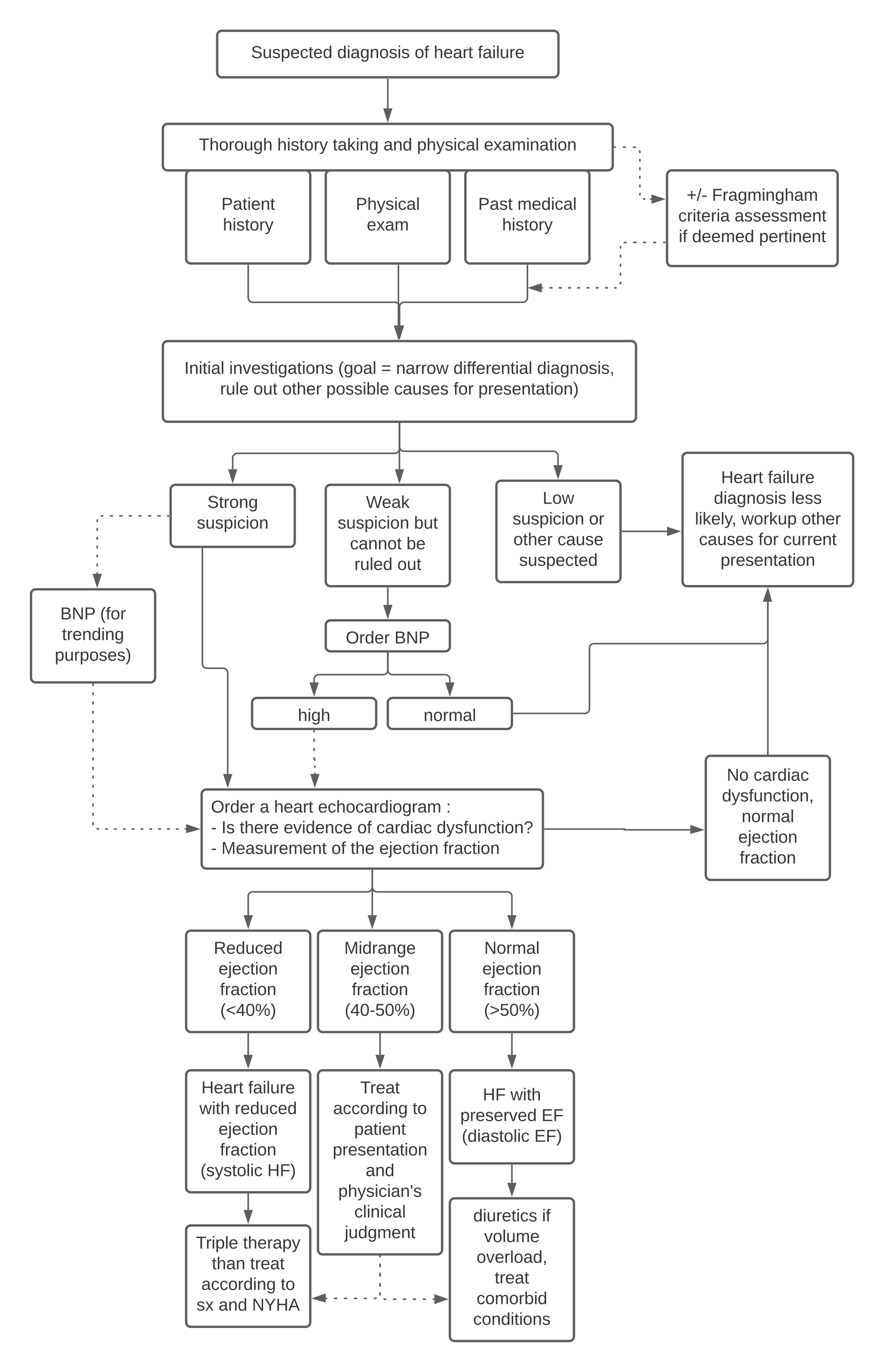
If clinical suspicion of HF remains high after the initial work-up, echocardiography should be ordered to confirm the diagnosis and determine ejection fraction. (1,2,5,8)
Beyond the Initial Approach
Many Presentations for the Same Disease
HF covers a wide variety of presentations, ranging from gradual lower extremity edema to rapid-onset respiratory distress. HF can present with acute or chronic symptoms. Acute or “decompensated” HF presents as an exacerbation of symptoms with or without a previous HF diagnosis. Severe acute HF can present with flash pulmonary edema and even cardiogenic shock. Chronic HF refers to patients with a diagnosis of HF whose condition and symptoms are currently stable. (7)
In a patient diagnosed with acute-on-chronic HF, precipitating factors should be investigated. The most common precipitating factors of acute exacerbations of HF are de novo or paroxysmal atrial fibrillation, acute myocardial infarction or ischemia, non-adherence to medication, new prescriptions impairing myocardial function (e.g., a calcium channel blocker), increased sodium and fluid intake, and physical overexertion. (7)
Pulmonary edema is defined as an abnormal buildup of fluids in the extravascular compartments of the lungs. In terms of pathophysiology, pulmonary edema is caused by severe left ventricular failure that leads to retrograde pulmonary venous hypertension. The chest X-ray findings suggestive of pulmonary venous congestion include cardiomegaly, interstitial edema, air bronchograms, Kerley B lines, and pleural effusions (see Figure 1). (7,8,9,10)
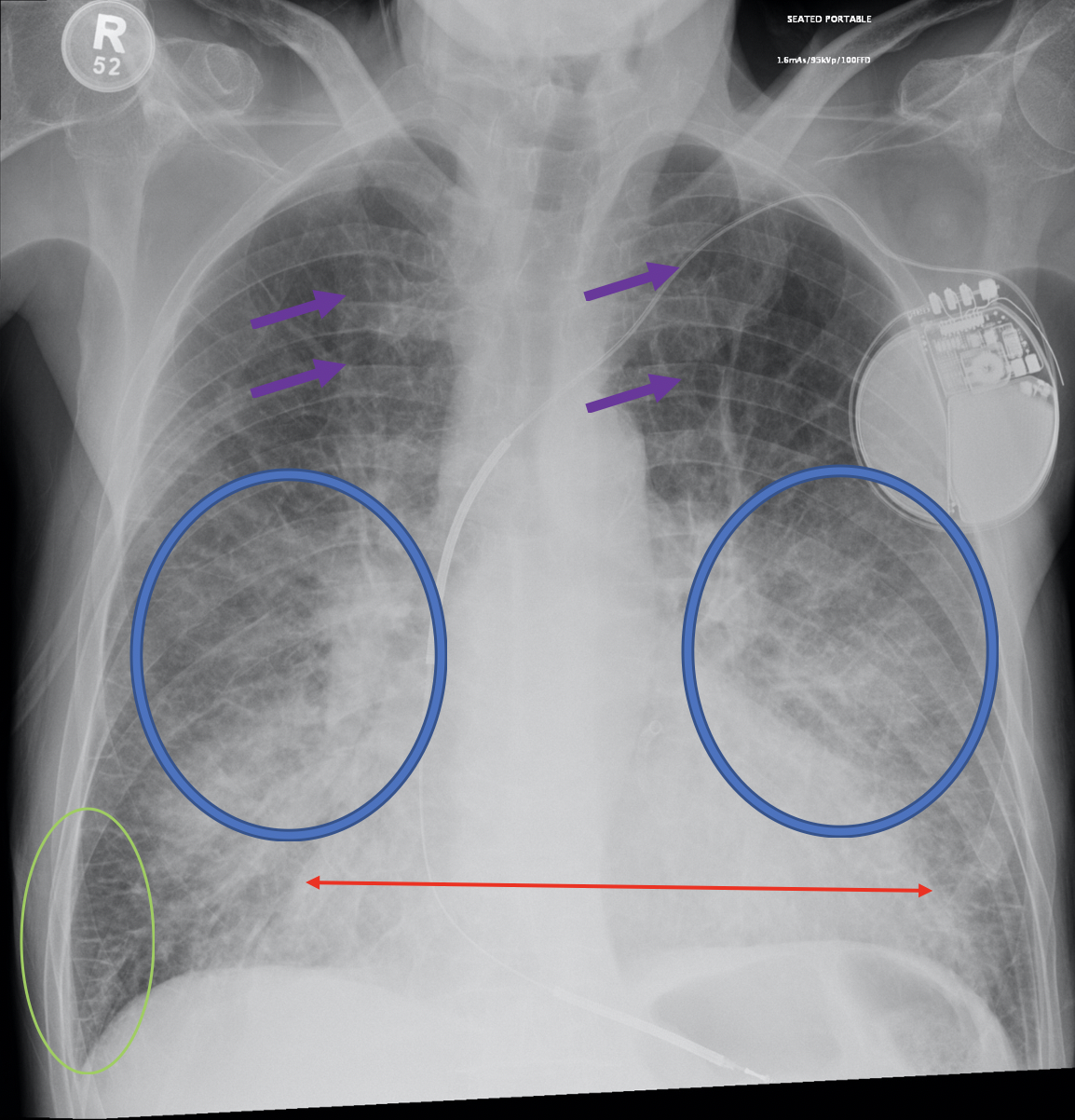
Purple arrows: upper lobes vascular redistribution, suggestive of pulmonary edema
Blue circles: increased interstitial lung markings suggestive of interstitial edema
Green circle: Kerley B-lines suggestive of interstital edema
Red arrow: cardiomegaly (increased cardiothoracic ratio)
Note this particular case does not display pleural effusions (costophrenic angles are clear)
Adapted from Radiopeda. Pulmonary Edema [Image on Internet]. Radiopedia; 2005-2020. Case courtesy of Dr Abeer Ahmed Alhelali; [cited 2020 Nov 6]. Available from: https://radiopaedia.org/cases/pulmonary-oedema-7
Cardiogenic shock is defined as a clinical state with evidence of tissue hypoperfusion and a systolic blood pressure <90 mm Hg. (7)
Preserved and Reduced Ejection Fraction
HF can be divided into two categories: HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). HFrEF and HFpEF are also sometimes referred to as systolic and diastolic HF, respectively. A reduced EF is defined as a LVEF of less than or equal to 40%. A preserved EF is defined as an LVEF of greater than or equal to 50%. An LVEF of between 40-50% is considered a “mid-range” or “borderline” EF and remains scarcely studied. (11) Nearly half of patients with HF have a preserved ejection fraction; it is therefore not a rare presentation. (5, 12, 13) HFpEF tends to be more common in women, older patients (> 65 years old), and in patients without a history of CAD. (5,6) EF cannot reliably be determined based on clinical presentation because signs and symptoms can be similar for both types of HF. (6)
Determination of EF has a two-fold purpose. Firstly, it is a strong predictor of the severity and prognosis of the disease. (2,14) The risk of all-cause mortality, as well as cardiovascular and congestive HF-related death, declines with increasing EF until around 45%. (10) Secondly, HFpEF and HFrEF do not share the same treatment. Therefore, adequate characterization of fraction ejection is essential to determine appropriate interventions.
Overview of Heart Failure Treatment
Heart failure treatment depends on ejection fraction. First-line treatment for HFrEF is a triple pharmacological therapy consisting of ACEi (or an angiotensin II receptor blocker (ARB) if ACEi intolerant), BB, and MRA. (1,2) Doses should be titrated to either evidence-based target doses or a maximum tolerated dose. In conjunction, diuretics should be prescribed and titrated to the lowest dose needed to maintain euvolemia. Non-pharmacological interventions include dietary sodium and fluid restriction; weight tracking; weight loss in obese patients; avoidance of alcohol, recreational drugs and tobacco; regular exercise; and seasonal vaccination. (15) Best practice also involves a discussion with the patient about their goals of care and their desired level of intervention. (1)
In the case of HFpEF, studies have shown no medications to be effective for a long-term reduction in mortality. (8,9) Thus, the main pillars of HFpEF management are centered on symptomatic treatment of fluid overload, comorbidities management, and tertiary prevention. Diuretics can be prescribed to reduce volume overload symptomatology. Non-pharmacological interventions can be recommended similarly to HFrEF. Blood pressure should be treated according to local guidelines. CAD and atrial fibrillation should be properly investigated and treated if present. Patients who can tolerate exercise should be referred to endurance and resistance training or cardiac rehabilitation. (12)
Acute HF should be treated similarly regardless of ejection fraction with O2 supplementation targeting saturation above 92%, intravenous furosemide (bolus or perfusion) for treatment of volume overload, and vasopressors if the patient has indications of shock. (1)
Judicious Use of BNP in HF
BNP is a hormone secreted by the ventricles of the heart in response to increased stretching or pressure. (16) It is often used as a marker for cardiac function. In the diagnosis of HF, BNP use is limited by its poor specificity despite its high sensitivity. Apart from HF, BNP levels can also be elevated due to acute or chronic renal failure, pulmonary embolism, acute respiratory distress syndrome, myocardial infarction, atrial fibrillation, sepsis, chemotherapy, and many more medical conditions. As such, BNP has a better negative than positive predictive value and should be used to rule out HF from the differential diagnosis rather than to rule it in. (11,12)
Once a diagnosis of HF is established, BNP can be used for monitoring purposes. While BNP often remains chronically high in patients with HF, a sudden rise can be a diagnostic clue to an exacerbation of the disease, especially if >30% of the patient’s baseline levels. (5)
Finally, the BNP level is a strong predictor of the risk of death and cardiovascular events in patients with HF. (11)
References
- Canadian Cardiovascular Society. Pocket Guide: Is it heart failure and what should I do? [Internet]. 2017 Updated Edition. Canada: Canadian Cardiovascular Society; 2017 [cited 2020 Oct 22]. 32p. Available from: https://www.ccs.ca/images/Guidelines/PocketGuides_EN/HF_Gui_2017_PG_EN_web.pdf
- King M, Kingery J, Casey B. Diagnosis and Evaluation of Heart Failure. Am Fam Physician [Internet]. 2012 Jun [cited 2020 Oct 22]; 85(12):1161-1168. Available from: https://www.aafp.org/afp/2012/0615/p1161.html#afp20120615p1161-t4
- Ezekowitz JA, O’Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol [Internet]. 2017 Nov [cited 2020 Oct 23]; 33(11):1342-1433. Available from: https://www.onlinecjc.ca/action/showPdf?pii=S0828-282X%2817%2930973-X doi: 10.1016/j.cjca.2017.08.022
- Public Health Agency of Canada. Report from the Canadian Chronic Disease Surveillance System: Heart Disease in Canada, 2018 [Internet]. Ottawa (CAN): Public Health Agency of Canada; 2018 May [cited 2020 Oct 23]. 70p. Available from: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/diseases-conditions/report-heart-disease-canada-2018/pub1-eng.pdf
- Lanthier. Insuffisance cardiaque [Internet]. Sherbrooke (CAN): Messil Inc.; [updated 2019; cited 2020 Oct 23]. Available from: https://app.lanthiermed.com/page/cardiologie/fr_insuffisance_cardiaque.html/
- Kannel, William. Framingham Heart Failure Diagnostic Criteria [Internet]. US:MDCalc; n.d. [cited 2020 Oct 23]. Available from: https://www.mdcalc.com/framingham-heart-failure-diagnostic-criteria
- Cydulka RK, Fitch MT, Joing SA, Wang VJ, Cline DM Ma JO. Tintinalli’s Emergency Medicine Maual. 8th ed. New York: McGraw Hill Education; 2017. Chapter 22, Acute Heart Failure; p. 149-151.
- Bell DJ, Weerakkody Y. Pulmonary Edema [Internet]. Radiopedia; 2005-2020. [cited 2020 Nov 6]. Available from: https://radiopaedia.org/articles/pulmonary-oedema
- Howlett JG. Pulmonary Edema [Internet]. Alberta: Merck’s Manual; 2020 Feb [cited 2020 Nov 6]. Available from: https://www.merckmanuals.com/professional/cardiovascular-disorders/heart-failure/pulmonary-edema
- Radiopedia. Pulmonary edema [image on internet]. 2005-2020 [cited 2020 Nov 6]. Available from: https://radiopaedia.org/cases/pulmonary-oedema-7
- Rastogi A, Novak E, Platts AE, Mann DL. Epidemiology, pathophysiology and clinical outcomes for heart failure patients with a mid-range ejection fraction. Eur J Heart Fail [Internet]. 2017 Dec [ cited 2020 Oct 23]; 19(12):1597-1605. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5730502/ doi: https://doi.org/10.1002/ejhf.879
- Gazewood JD, Turner PL. Heart Failure with Preserved Ejection Fraction: Diagnosis and Management. Am Fam Physician [Internet]. 2017 Nov [cited 2020 Oct 23]; 96(9):582-588. Available from: https://www.aafp.org/afp/2017/1101/p582.html
- Mayo Clinic. Heart failure with preserved ejection fraction (HFpEF): More than diastolic dysfunction [Internet]. US: Mayo Clinic; 2017 May [cited 2020 Oct 23]. Available from https://www.mayoclinic.org/medical-professionals/cardiovascular-diseases/news/heart-failure-with-preserved-ejection-fraction-hfpef-more-than-diastolic-dysfunction/mac-20430055
- Solomon SD, Anevekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, et al. Influence of Ejection Fraction on Cardiovascular Outcomes in a Broad Spectrum of Heart Failure Patients. Circulation [Internet]. 2005 Dec [cited 2020 Oct 28] 112(24):3738-44. Available from: https://www.ahajournals.org/doi/epub/10.1161/CIRCULATIONAHA.105.561423 doi: 10.1161/CIRCULATIONAHA.105.561423
- Gibbs CR, Jackson G, Lip GYH. Non-drug Management. BMJ [Internet]. 2000 Feb [cited 2021 Jan 11] 320(7231): 366–369. doi: 10.1136/bmj.320.7231.366
- Doust J, Lehman R, Glasziou P. The Role of BNP Testing in Heart Failure. Am Fam Physician [Internet]. 2006 Dec [cited 2020 Nov 1] 74(11):1893-1900. Available from: https://www.aafp.org/afp/2006/1201/p1893.html

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.