Approach to
Gynecological Adnexal Masses
Laurie-Rose Dubé1
Published online: 20 July 2021
1Faculty of Medicine, McGill University, Montreal, QC, Canada
Corresponding Author: Laurie-Rose Dubé, email laurierose.dube@hotmail.com
DOI: 10.26443/mjm.v20i2.340
Abstract
Gynecological pelvic masses are a common occurrence in women of all ages. The differential diagnosis is extensive and includes masses of all anatomical components of the female reproductive tract. This simple and refined approach leads the reader through the process of narrowing said differential. A thorough history and physical examination are essential steps that can hint to the appropriate investigations such as reproductive hormone levels, serum cancer biomarkers and imaging. Emphasis is put on ultrasound findings, helping differentiate not only diagnoses, but also the benign or malignant character of the mass. It also highlights the Risk of Malignancy Index I, commonly used in clinical practice to assess the risk of malignancy of a mass. Beyond the initial approach, some diagnoses and their management are discussed, from the very common functional cyst to the worrisome ovarian neoplasm, and mentioning more peculiar findings like tubo-ovarian abscess and leiomyoma.
Tags: Pelvic mass, Gynecological mass
Question
A 50-year-old female presents to a gynecology clinic after her family physician palpated a mass on her right ovary during a routine physical examination. The patient began menopause a year ago. For 4 months, she has been experiencing fatigue, decreased appetite and bloating, but believes those symptoms are due to the hormonal changes associated with menopause. She doesn’t report significant weight loss or other symptoms such as fever, nausea, vomiting, or bladder or bowel dysfunction. She also doesn’t report any abdominal pain, uterine bleeding or abnormal vaginal discharge. She has no history of cancer, and her family history is unknown.
Her past medical and surgical histories are unremarkable aside from mild hypertension. The patient is not known for any gynecological conditions such as other pelvic masses, endometriosis or sexually transmitted infections. Her gynecological history is G3P2A1 with two normal vaginal deliveries and one unexplained first-trimester miscarriage.
The patient doesn’t smoke or use any recreational drugs but drinks socially twice a month. She is sexually active with one male partner and uses condoms as protection. She doesn’t take any medications and is making lifestyle modifications to control her mild hypertension.
Her physical examination is normal, including the speculum examination of the cervix. During the bimanual pelvic exam, a solid, fixed and irregular mass is palpated on her right ovary. The mass is estimated to measure 4 or 5 centimeters. Rectovaginal exam is not performed. The following investigations are performed:
Blood test
Complete Blood Count (CBC) within normal limits
Serum CA-125: 44 units/mL (normal <35 units/mL)
Transvaginal sonography with Doppler
6 cm irregular mass on right ovary with multiple thickened septa and a large solid component. No ascites. Doppler shows high blood flow (colour score 4).
Uterus and left adnexa appear normal.
Risk of Malignancy Index I (RMI)
RMI= 396 (normal <250)
What is the next best step to take for the management of this patient?
- MRI
- Refer to gynecological-oncologist.
- Mass tissue biopsy
- Surgical resection
- Regional lymph node biopsy
Answer
B. The CBC is normal and shows no sign of infection. The serum cancer antigen 125 (CA-125) is elevated, but the specificity of this tumor marker is 71-93% and other conditions, such as endometriosis, could be responsible for its elevation. Thus, further investigations are necessary to assess the risk of malignancy. The ultrasound findings (irregularity, nodularity and solidity), the patient’s menopausal state and positive CA-125 all contribute to the elevated RMI, which is used to predict the potential malignancy of an adnexal mass. Taken together, the provided information raises suspicion for malignancy. In this context, the proper management would be referral to a gynecological-oncologist.
Initial Approach
The evaluation of a woman with an adnexal mass begins by eliciting a complete history from the patient, including a sexual and gynecological history, accompanied by a physical exam with pelvic exam and speculum. (1, 2) Most women presenting with an adnexal mass are asymptomatic and, consequently, the mass is often an incidental finding. (1) In the cases where symptoms are present, they vary greatly according to the location and size of the mass; the most common symptom being pelvic pain. (1) While history and physical examination are essential and can significantly narrow the differential diagnosis, imaging is almost always necessary for definite diagnosis (Fig. 1). (1, 2)
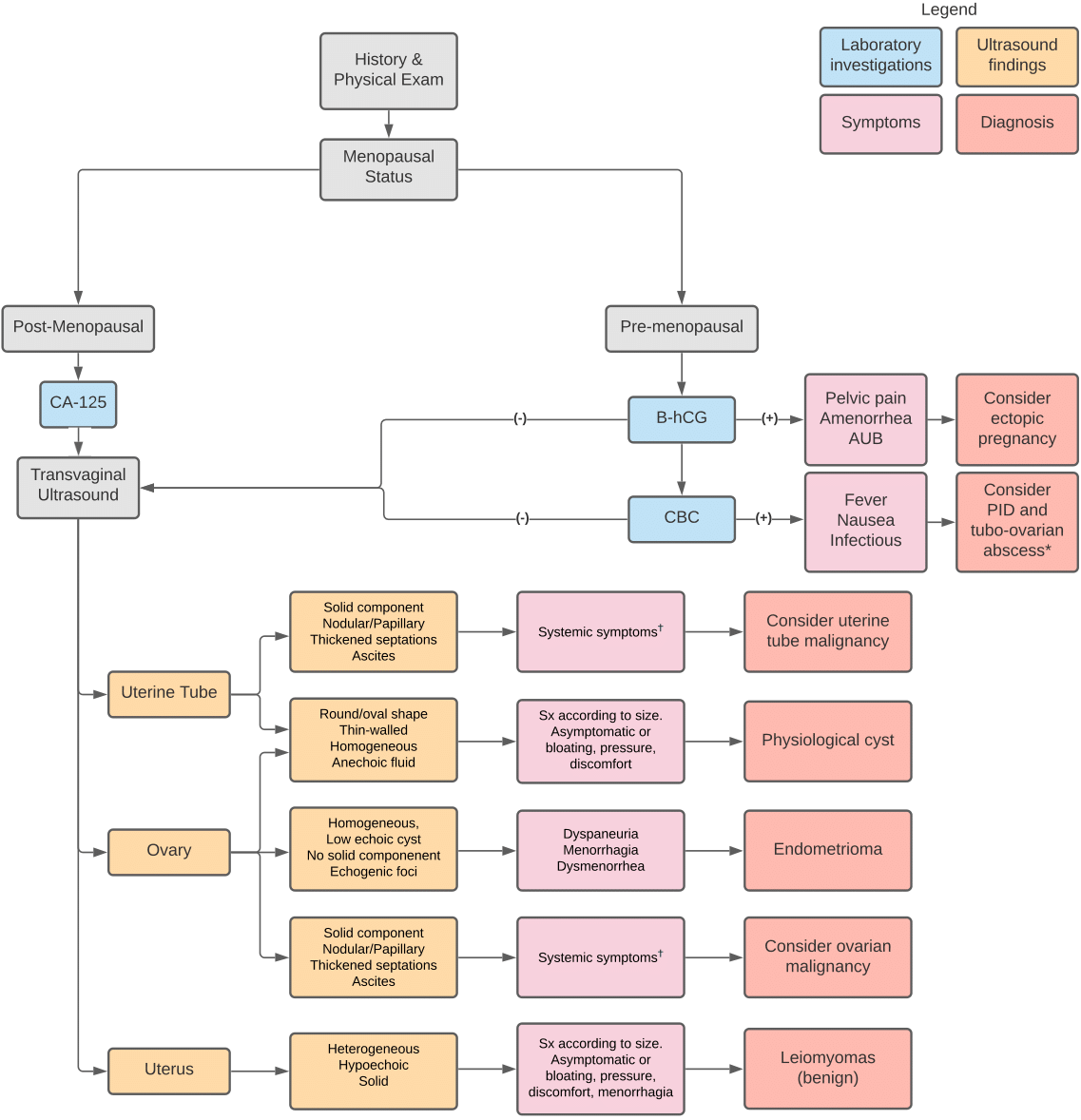
This basic algorithm represents the usual management of a patient who presents with a gynecological mass. Including the patient’s history and basic laboratory investigations, it is centered on the ultrasound findings which is the modality of choice in the evaluation of a pelvic mass. Common symptoms of specific conditions are indicated but it is crucial to remember that most pelvic masses are asymptomatic.
*Although they are more prevalent in premenopausal women, pelvic inflammatory disease and tubo-ovarian abscesses can also present rarely in post-menopausal women.
†Adnexal malignancies can also be asymptomatic or present with symptoms associated with their size, notably bloating, pressure, discomfort and urinary symtpoms.
AUB: Abnormal uterine bleeding
B-hCG: Beta-human chorionic gonadotropin
CA-125: Cancer antigen 125
CBC: Complete blood count (Note: in this flowchart, it is said to be positive if it shows signs of infection, e.g. elevated white blood cell count)
PID: Pelvic inflammatory disease
Sx: Symptom
History and symptoms
The differential diagnosis for an adnexal mass is broad and although ultrasound is the most efficient diagnostic method, a thorough history including an assessment of any pain and bleeding can narrow the differential substantially. (1, 2, 4) Pelvic pain, amenorrhea, and abnormal uterine bleeding are suggestive of an ectopic pregnancy. (1) A patient with an ectopic pregnancy can present with tachycardia, hypotension and hemodynamic instability. (2) Dyspareunia, dysmenorrhea and pelvic or abdominal pain are indicative of endometriosis. (1, 2) Pelvic pain associated with fever, nausea, vomiting and vaginal discharge is suggestive of pelvic inflammatory disease or of a fallopian abscess. (1) Abnormal menses can also hint to different conditions; for example, dysmenorrhea or menorrhagia could be suggestive of uterine leiomyomas, while oligomenorrhea and hirsutism are more indicative of polycystic ovarian syndrome (PCOS). (1) In general, the increasing size of a mass is linked to the apparition of symptoms as adjacent structures are compressed. (2) A significant mass such as a fibroid can cause abdominal distension, nausea, vomiting, and bowel or bladder dysfunction. (1, 2) A malignant mass, as it enlarges, can also exhibit these symptoms in addition with canonically recognized systemic symptoms such as fatigue, fever, weight loss, and night sweats. (1, 2, 3)
Blood tests and tumor markers
Blood testing is not always necessary in the setting of an adnexal mass. (1) In general, women of reproductive age should be tested for serum beta-human chorionic gonadotropin (ß-hCG) in order to rule out pregnancy. (1) In the event pregnancy is confirmed, ectopic pregnancy must be investigated. (1) If the patient presents with fever and signs of infection, a complete blood count is ordered to confirm infection which can point to pelvic inflammatory disease (1) or to a tubo-ovarian abscess. (2) In both of these conditions, WBC count is elevated with a predominance of neutrophils. (2) Tumor markers can raise suspicion for the malignancy of a mass, but they should never be considered as a diagnostic criterion. (1, 3) Cancer antigen 125 (CA-125) is a serum biomarker commonly assessed in post-menopausal women presenting with a symptomatic mass and is associated with epithelial ovarian cancer (OEC). (4, 5) However, specificity is low in early-stage malignancy and in pre-menopausal women; CA-125 can be elevated by multiple physiologic and pathologic conditions such as pregnancy, endometriosis, fibroids and pelvic inflammatory disease. (1, 5) Other tumor markers can be evaluated depending on the patient’s age and the characteristics of the mass. In women under the age of 40 years old, tumor markers such as hCG, lactate dehydrogenase, alpha-fetoprotein, and inhibin should be included to assess for rare tumors such as germ cell tumors and sex-cord stromal tumors. (4) Women presenting with bilateral masses or with suspected tumor metastases should be evaluated for tumor markers such as carcinoembryonic antigen, cancer antigen 19-9, and cancer antigen 15-3. (4)
Transvaginal sonography (TVS)
Sonography is the imaging modality of choice when it comes to adnexal masses, as it allows the physician to locate and characterize the mass. (1, 2, 3) TVS should be performed in combination with color Doppler ultrasound. (4) Location and characteristics of the mass can be highly suggestive of a diagnosis. Simple cysts, which can be ovarian, paraovarian or paratubal, are non-solid and the transmission of ultrasound signals will not be impaired; therefore, they appear as homogeneous round or oval thin-walled cysts with anechoic fluid in the cavity (Fig. 2A). (6, 7, 8) The finding of a cyst accompanied by a reticular pattern of thin echoes is suggestive of hemorrhage (Fig. 2B). (6, 8) Dermoid cysts present with hyperechoic nodules within the mass with distal acoustic shadowing (Fig. 2C). (6, 8) Dermoid cysts are benign germ cells tumors, also called mature teratomas, and can contain various tissues such as hair, teeth and sebum. (6) Ultrasound can also be employed to characterize other common benign masses; endometrioma is a homogeneous, low echoic cyst without a solid component, but with a small echogenic focus on the inner wall of the cyst (Fig. 2D). (6, 7, 8) Leiomyomas, also called fibromas or fibroids, are benign smooth muscle tumors and appear as heterogeneous, hypoechoic, solid masses (Fig. 2E). (6, 7, 8) Cysts and masses are more likely to be malignant when they have the following features: solid non-hyperechoic component, nodularity or papillary structures, thickened septations, associated ascites in the rectouterine pouch, and high vascularity on Doppler (Fig. 2F). (4, 6, 8) Masses exhibiting these features are often associated with ovarian or uterine tube malignancy. (6, 8)
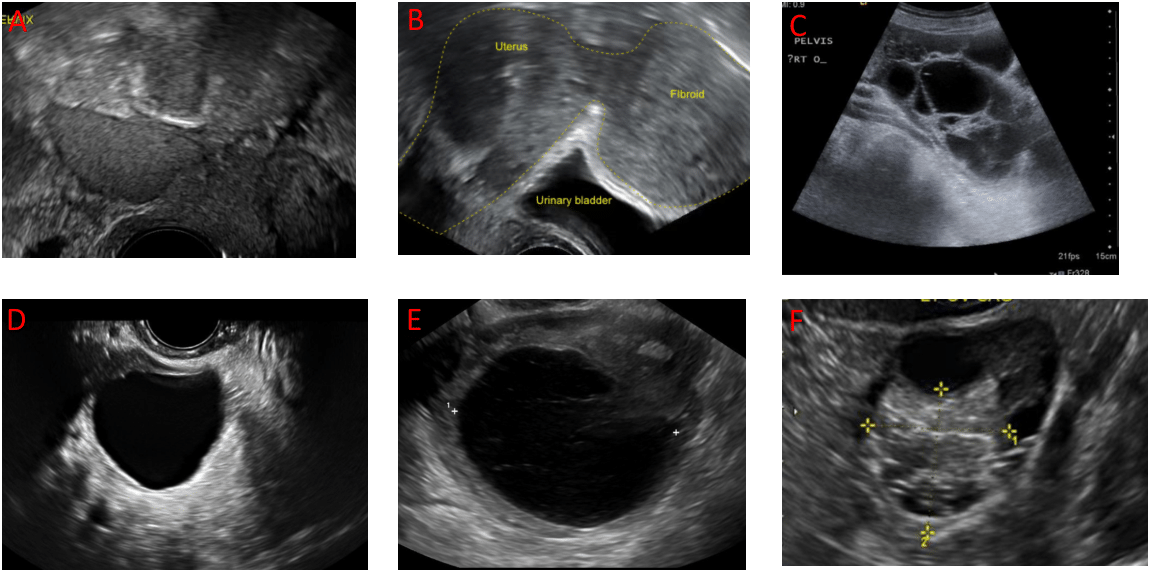
- Simple cyst: thin-walled, no septations, unilocular, fluid-filled. Reproduced with permission of the Cleveland Clinic Center for Continuing Education. Ross E, Fortin C. Ovarian Cysts. Disease management (http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/womens-health/ovarian-cysts/). ©2000-2020 The Cleveland Clinic Foundation. All rights reserved.
- Hemorrhagic cyst: reticulated pattern within the cyst. https://upload.wikimedia.org/wikipedia/commons/3/35/Haemorrhagic\_ovarian\_cyst\_ultrasound.jpg
- Dermoid cyst (mature teratoma): hyperechoic nodules, acoustic shadowing, presence of abnormal tissue (fat, hair, sebum, teeth, etc...). Reproduced with permission of the Cleveland Clinic Center for Continuing Education. Ross E, Fortin C. Ovarian Cysts. Disease management (http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/womens-health/ovarian-cysts/). ©2000-2020 The Cleveland Clinic Foundation. All rights reserved.
- Endometrioma : homogeneous, low to medium echoic, no solid component or nodules. Reproduced with permission of the Cleveland Clinic Center for Continuing Education. Ross E, Fortin C. Ovarian Cysts. Disease management (http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/womens-health/ovarian-cysts/). ©2000-2020 The Cleveland Clinic Foundation. All rights reserved.
- Subserosal pedunculated uterine fibroid: heterogeneous, hypoechoic, solid. https://commons.wikimedia.org/wiki/File:Subserosal\_uterine\_fibroid.png
- Ovarian cyst: multilocular, non-hyperechoic solid areas, thick septations, ascites, other potential masses. Strong suspicion for malignancy. Reproduced with permission of the Cleveland Clinic Center for Continuing Education. Ross E, Fortin C. Ovarian Cysts. Disease management (http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/womens-health/ovarian-cysts/). ©2000-2020 The Cleveland Clinic Foundation. All rights reserved.
International Ovarian Tumor Analysis (IOTA) – Simple rules
The IOTA group developed a model of simple rules to help distinguish benign and malignant masses on sonography. The IOTA model is useful to all physicians and is more specific and sensitive than other predictive tools like the RMI. The simple rules are comprised of 5 features that are suggestive of a malignant mass (M-features) and 5 features that are suggestive of a benign mass (B-features). M-features include:
M1 – irregular solid tumor
M2 – presence of ascites
M3 – presence of 4+ papillary structures
M4 – irregular multilocular solid tumor with a diameter =100mm
M5 – high vascularization (Doppler score of 4).
B-features include:
B1 – unilocular cyst
B2 – presence of solid components with a diameter <7mm
B3 – presence of acoustic shadows
B4 – smooth multilocular tumor with a diameter <100 mm
B5 – no vascularization (Doppler score of 1) (Fig. 3).

as seen at https://www.iotagroup.org/education/educational-material
If a mass has one or more B-features and no M-features, the mass is classified as benign. Alternately, a mass with one or more M-features and no B-features is classified as malignant. Up to 25% of masses are indeterminate, meaning that both B-features and M-features are present or that none of the rules apply. Indeterminate masses should be reassessed by an expert sonographer and malignant masses should be immediately referred to a gynecological-oncologist. (4)
Risk of Malignancy Index I (RMI)
RMI I is currently used as a clinical prediction rule for malignancy risk evaluation of an adnexal mass. (1, 3, 5) It is comprised of three variables: ultrasound (U), menopausal status (M) and serum CA-125. (5) Ultrasound findings such as multilocular cysts, solid areas, metastases, ascites and bilateral lesions are taken into consideration. (1, 5) Absence of these characteristics corresponds to U=0, one finding corresponds to U=1 and two to five findings corresponds to U=3. M is the menopausal status; pre-menopausal is denoted by M=1 and post-menopausal is denoted by M=3. (1, 5) The final variable directly represents CA-125 serum concentration in units/mL. (1, 5) In order to obtain the RMI, all three variables must be multiplied (1, 5); for example, a post-menopausal woman with bilateral multilocular ovarian cysts and a CA-125 of 49 units/mL would obtain an RMI I score of U(3) x M(3) x CA-125(49) = 441. The cutoff for referral to an oncologist is for any RMI = 250. (1, 5) However, many physicians refer when the RMI = 200 due to a high suspicion of malignancy and the necessity of performing more targeted investigations. (1, 5)
Beyond the initial approach
This section covers the most common diagnoses, the ones not to miss, and their basic management.
Functional cysts
Functional cysts are by far the most common diagnosis. (1) They are physiological and will often spontaneously resolve, which is why they are said to be “functional” or “organic”. (1, 8) They include follicular cysts, hemorrhagic cysts, corpus luteal cysts, and theca-lutein cysts. (1, 2) Cysts with a classic benign presentation should be followed on ultrasound in 8 to 12 weeks and then yearly for up to 5 years or until resolution. (9) Their associated risk of malignancy is extremely low, but a cyst that hasn’t resolved, has developed malignant features, or has grown over more than 6 months should raise suspicion and may require further investigations. (1, 2, 3, 9)
Ovarian cancer
Ovarian cancer is the second most common gynecological malignancy. (1) There is no screening for ovarian cancer and it is often discovered during the later stages at which it becomes symptomatic. (1,10) 90% of ovarian cancer are epithelial carcinomas for which CA-125 is the main identified biomarker. (1) RMI I results can point to ovarian malignancy but diagnosis should always be confirmed by biopsy, either laparoscopic or image-guided. (10) The prognosis depends on the type of cancer, its stage, and grade. (1) In mild cases, surgical debulking and lymph node sampling are common therapeutic strategies. (1) However, total hysterectomy and bilateral salpingo-oopherectomy are almost always recommended, sometimes accompanied by adjuvant chemotherapy of carboplatin and paclitaxel. (1, 10) It is crucial to mention that ovarian cancer can be associated with a BRCA1/BRCA2 mutation, as well as hereditary nonpolyposis colorectal cancer, and that genetic testing might be advisable in patients presenting with a family history compatible with these cancers. (1)
Tubo-ovarian abscess (TOA)
A TOA is an inflammatory fallopian mass often accompanied by symptoms such as fever, nausea, vomiting, and purulent discharge. (1, 11) They are most common in reproductive age patients and are a complication of pelvic inflammatory disease (PID), which is often caused by sexually transmitted pathogens or bacterial vaginosis-related pathogens. (11) PID is an acute infection of the upper genital tract and thus should be treated with an antibiotic regimen that usually includes Cefotetan IV and Doxycyline IV/PO. (11) If the patient is found to be unresponsive to therapy, drainage and surgery should be considered. (11)
Adnexal leiomyomas
Leiomyomas, or fibroids, are benign neoplasms arising from smooth muscles. (12) They can be found in the uterus or in the broad ligament. (12) They are usually asymptomatic unless they are large (bloating, compression, pain) or intra-uterine (menorrhagia, dysmenorrhea). (1, 12) The pain can be treated with NSAIDs and the patient may be prescribed oral contraceptives. (12) Surgical treatments are also available for the symptomatic patient, including uterine artery embolization, and in extreme cases, myomectomy or hysterectomy. (12)
References
- Adnexal Mass. In: EBSCO Information Services. DynaMed [database on the Internet]. Ipswich (MA): DynaMed, 2018 [cited 2020 Sep 9]; T115395. Available from https://www.dynamed.com/topics/dmp~AN~T115395.
- Biggs WS, Marks ST. Diagnosis and management of adnexal masses. American family physician. 2016 Apr 15;93(8):676-81.
- vanSchagen JE. Pelvic mass. In: Smith MA, ed. Essential Evidence Plus [database on the Internet]. Hoboken (NJ): John Wiley & Sons, Inc; 2020 [cited 2020 Sep 9]. Available at https://www-essentialevidenceplus-com.proxy3.library.mcgill.ca/content/eee/245
- Salvador S, Scott S, Glanc P, Eiriksson L, Jang JH, Sebastianelli A, Dean E. Guideline No. 403: Initial Investigation and Management of Adnexal Masses. Journal of Obstetrics and Gynaecology Canada. 2020 Aug 1;42(8):1021-9.
- Dochez V, Caillon H, Vaucel E, Dimet J, Winer N, Ducarme G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1): 28.
- Patel MD. Ultrasound differentiation of benign versus malignant adnexal masses. UpToDate [database on the Internet]. Waltham (MA): UpToDate, 2020 [cited 2020 Sep 9]. Available from https://www. uptodate. com/contents/ultrasound-differentiation-of-benign-versus-malignant-adnexal-masses.
- Alessandrino F, Dellafiore C, Eshja E, Alfano F, Ricci G, Cassani C, La Fianza A. Differential diagnosis for female pelvic masses. Medical Imaging in Clinical Practice. 2013 Feb 20:222-30.
- Ross E, Fortin C. Ovarian Cysts. Available from: http://www.clevelandclinicmeded.com/medicalpubs/ diseasemanagement/womens-health/ovarian-cysts/
- Wolfman W, Thurston J, Yeung G, Glanc P. Guideline No. 404: Initial Investigation and Management of Benign Ovarian Masses. Journal of Obstetrics and Gynaecology Canada. 2020 Aug 1;42(8):1040-50.
- Le T, Giede, C. Initial evaluation and referral guidelines for management of pelvic/ovarian masses. JOGC. 2018;40(3); 223-229.
- Beigi RH. Management and complications of tubo-ovarian abscess. UpToDate [database on the Internet]. Waltham (MA): UpToDate, 2020 [cited 2020 Sep 9]. Available from https://www.uptodate.com/contents/management-and-complications-of-tubo-ovarian-abscess.
- Stewart EA. Uterine fibroids. New England Journal of Medicine. 2015 Apr 23;372(17):1646-55.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.