Approach to
Management of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia
David Bouhadana1, Iman Sadri1
Published online: 1 July 2021
1Faculty of Medicine and Health Sciences, McGill University, Montreal, QC, Canada
Corresponding Author: David Bouhadana, email david.bouhadana@mail.mcgill.ca
DOI: 10.26443/mjm.v20i2.330
Abstract
Benign prostatic hyperplasia (BPH) is a condition that affects up to 50% of men over the age of 50; the condition’s prevalence increases with age, particularly after the age of 40. (1, 2) BPH can lead to lower urinary tract symptoms (LUTS) which can have a significant negative impact on health-related quality of life (HRQoL). (2-4) Men presenting with a gradual onset of LUTS are often suspected to have BPH. However, the clinician must recognize that LUTS possess many different aetiologies. This article aims to provide medical students with a stepwise approach to the diagnosis and management of LUTS that are secondary to BPH. The outlined approach describes the differential diagnoses, required investigations, and management-related details for LUTS that are secondary to BPH. This approach is based off of relevant Canadian, American, and European urological association guidelines.
Tags: BPH, LUTS
Question
A 54-year-old male presents to your clinic with a concern about his urinary symptoms. He reports having to wake up 2 to 3 times per night to urinate, having a very weak stream of urine, and often feeling incompletely voided after urination. The patient has a family history of prostate cancer. He denies taking any medication or using any recreational drugs. The patient’s past medical history is notable for alcoholic hepatitis and hypertension; both are well controlled.
At this current stage, which of the following investigations would you not consider?
- A formal symptom inventory
- Prostate biopsy
- Serum prostate specific antigen (PSA)
- Urinalysis
- Digital rectal exam (DRE)
Answer
- A formal symptom inventory such as the International Prostate Symptom Score is recommended at initial presentation. This score allows physicians to objectively assess and keep track of the patient’s symptom severity when proposing treatment options. (5)
- Although a family history of prostate cancer increases a patient’s risk for prostate cancer, he is not known to have a palpable mass or elevated PSA levels. Therefore, a prostate biopsy does not need to be performed at this moment. (5, 6)
- Serum PSA is a low-cost and non-invasive test that can be used as a surrogate for prostate size. PSA can be helpful for the detection of prostate cancer, and the test can be offered to all males beginning at age 50 (or beginning at age 40 for patients with risk factors of prostate cancer) with at least 10 years of life expectancy. Therefore, given the patient’s age and his family history of prostate cancer, ordering a PSA test would be helpful given proper counselling and the employment of shared decision making. (5)
- Performing a urinalysis can help in ruling out urinary tract infections, hematuria, urothelial carcinomas, and bladder or kidney stones. All of these disorders should be included in the patient’s differential diagnosis. (5)
- Given that the patient’s presentation is suggestive of urinary obstruction, a DRE would be helpful to assess prostate size and to rule out the presence of nodular irregularities (suggestive of cancer) or tenderness (suggestive of infection/inflammation). (5)
Initial Approach
Patients presenting with a gradual onset of lower urinary tract symptoms (LUTS) are often suspected to have benign prostatic hyperplasia (BPH). However, the clinician must recognize that LUTS can be caused by many different conditions.
Patient History and Physical Examination
In order to rule out the most concerning aetiologies when diagnosing BPH, the proposed approach recommends beginning with a thorough history and complete physical examination. (5, 9, 10) The history should provide a detailed description of onset, duration, and severity for the patient’s LUTS. The medication history is especially important in the diagnosis of LUTS because many medications, such as antidepressants, diuretics, bronchodilators, and antihistamines, are associated with LUTS. (11)
Additionally, if suspected, the clinician must investigate for a history of:
- Urethral trauma - suggestive of urethral stricture
- Gross hematuria - suggestive of bladder stones, BPH, or cancer
- Underlying neurologic diseases - suggestive of neurogenic overactive bladder
- Diabetes mellitus
- Cigarette smoking - an important risk factor for bladder cancer
The physical exam should include a digital rectal examination (DRE) and an assessment for bladder distention and neurologic impairment. (9, 10) The DRE can be helpful for two reasons: it is an initial screening method for prostate cancer (PCa) and it can serve to roughly estimate prostate volume. (9) Once DRE is complete, the differential diagnoses described in the Flowchart 1 should be further explored, depending on the physician’s clinical suspicion, in order to rule out other causes of LUTS.
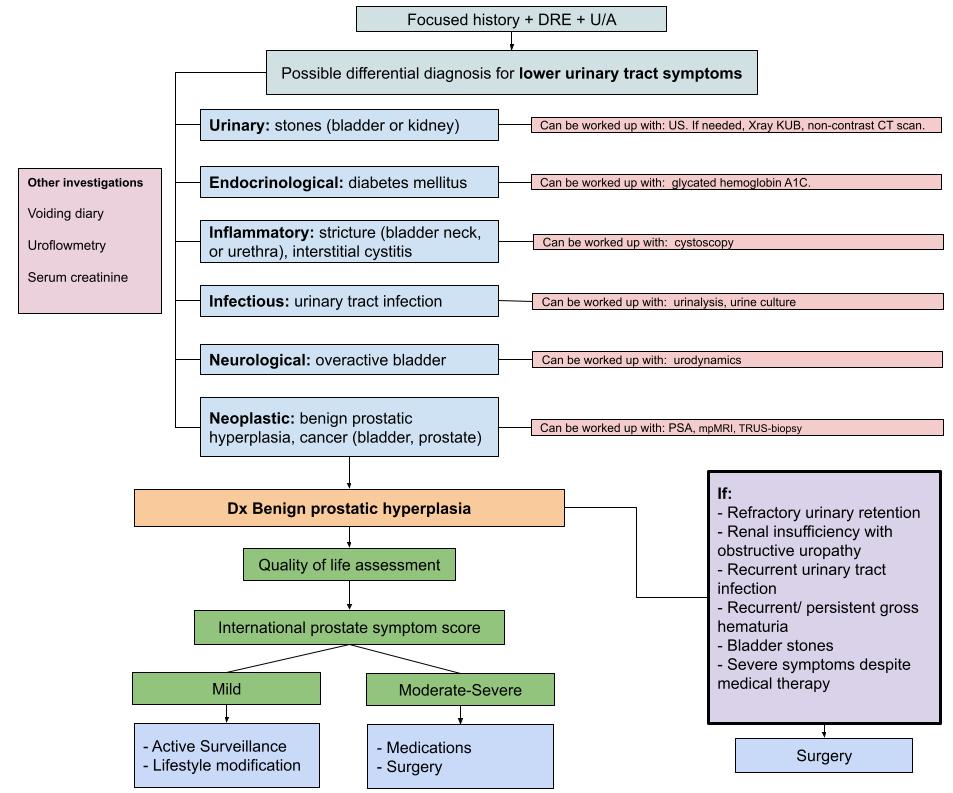
Treatment algorithm for the diagnosis and management of lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH). The diagnosis of BPH can be made with a focused history, DRE, and urinalysis in the absence of signs/symptoms of other causes of lower urinary tract symptoms. Further investigations for other possible causes of LUTS are briefly mentioned. The management of BPH relies on the patient’s LUTS severity and the impact that they have on the patient’s quality of life. Importantly, the recommended investigations included in this proposed approach are usually ordered concomitantly rather than in a step-wise fashion.
Recreated from Badalato et al. (2020). (7)
Differential Diagnosis
In the case of LUTS, one of the most important conditions on our differential diagnosis is PCa. Patients are usually screened for PCa after careful counselling with their urologist because most cases of PCa (67%) are considered to be clinically insignificant and do not have an impact on a patient’s morbidity and mortality. (12) Pathologically, PCa may present as a mass in the peripheral zone of the prostate and can be easily felt during a DRE. Therefore, if a mass is felt upon palpation it can be indicative of PCa. Due to their peripheral localization, these masses are often not associated with urinary symptoms. However, if a large, localized tumor is present, it can compress the urethra and consequently lead to LUTS. Despite the fact that most patients diagnosed with PCa will experience clinically insignificant disease, it is important to rule out PCa, as it can be life-threatening in specific cases. Importantly, the specificity of DRE is not absolute; patients diagnosed with PCa may present with no positive findings upon DRE.
If the patient does not have a family history of PCa, and the DRE does not reveal any prostatic masses, PCa cannot be ruled out in patients presenting with LUTS. The next recommended investigation would be to request a serum prostate specific antigen (PSA) analysis (5, 9, 10, 13). Although some controversy exists, the Canadian Urological Association recommends measuring the PSA level in patients presenting with LUTS that would benefit from treatment of PCa if the former were to be detected with further investigation (i.e. patients with greater than 10 years of life expectancy). Nguyen et al. recommend that PSA testing be carried out through a shared decision-making process because the perceived benefits and harms of PCa treatments may vary within the patient population. (14) As implied by its name, the PSA is prostate specific and not PCa specific. In fact, numerous conditions can lead to an elevated PSA such as: BPH, prostatitis, recent ejaculation, recent DRE, recent urethral instrumentation, and PCa. (5) If both a DRE and PSA yield results suggestive of PCa, the patient should be further worked up for PCa with the help of magnetic resonance imaging (MRI) and guided prostate biopsy. (15)
In the cases where PCa is ruled out, a urinary tract infection (UTI) should be ruled out because it is a more common cause of LUTS. To rule out a UTI, urinalysis can be performed. (9, 10, 13) According to Alawamlh et al. urinalysis is strongly suggested to help rule out hematuria, pyuria, and bacteriuria which are all relevant aetiologies of LUTS. (16) The clinician must remember that in practice these investigations can be ordered concomitantly rather than in a step-wise fashion. Therefore, PCa may not be ruled out by the time the patient is sent for UTI investigations.
Additional and less invasive investigations can be ordered initially, and these include a voiding diary, serum creatinine, and uroflowmetry. If the aforementioned examinations did not help identify the cause of LUTS, the following investigations can be performed for patients with concomitant diseases and/or uncertain diagnoses: measuring post-void residual volume, urodynamics, radiological evaluation of the urinary tract, and a sexual function questionnaire. (5) The latter investigations listed can help rule out causes of LUTS such as overactive bladder (OAB), urethral stricture, and bladder/kidney stones. (9, 10)
Symptom Score Assessment
At this stage of the proposed approach, the patient’s LUTS are likely assumed to be secondary to BPH. Once a diagnosis of BPH has been confirmed, the patient’s LUTS must be reassessed and quantified with the help of the IPSS. (5) The IPSS is a validated questionnaire that quantifies the severity of patients’ LUTS such that physicians can treat their symptoms accordingly. (17)
In addition to the seven questions of the IPSS survey, physicians often inquire as to the patient’s degree of bother related to their LUTS. This assessment is done with the help of the IPSS-QoL which consists of an additional question that asks patients to rate how they would feel if they were to spend the rest of their life with their LUTS. The score ranges from 0 (delighted) to 6 (terrible quality of life). This question can further assist physicians in determining the appropriate intervention for the patient, using a patient-centred approach. This facet is important because the treatment of BPH is aimed at symptom management and is guided by symptom severity, their degree of bother, and patient preferences. (5) A detailed presentation and description of the IPSS and the IPSS-QoL can be found in Table 1.
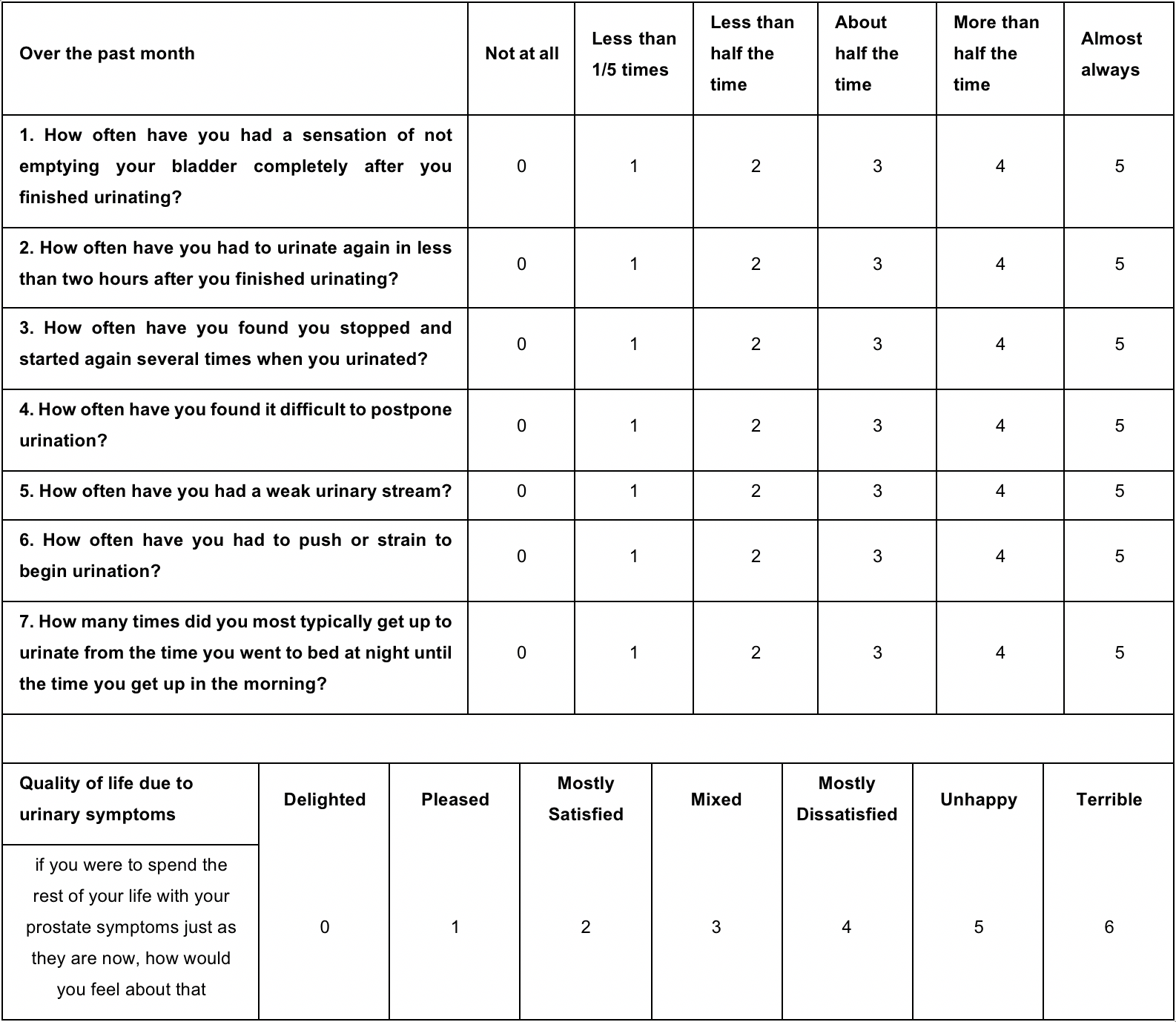
The International Prostate Symptom Score (IPSS) or American Urological Association Symptom Index is a validated symptom questionnaire used to assess symptom severity in patients affected by LUTS (8). An additional question related to quality of life is usually added to the IPSS to help evaluate the degree to which a patient is bothered by LUTS.
Retrieved from Barry et al. (8)
Following the completion of the IPSS, patients will be categorized according to their symptom severity in the following groups:
- Mild symptoms: scores of 0-7
- Moderate symptoms: scores of 8-19
- Severe symptoms: scores of 19-35
Management
Given its benign nature, BPH does not require immediate treatment in the absence of clear indications, such as refractory urinary retention or renal insufficiency caused by obstructive uropathy. However, if left untreated, BPH can lead to acute urinary retention which can increase the risk of UTIs, bladder stones, and renal damage. (9)
Often, patients with mild LUTS will improve over time without treatment. According to society guidelines, it is not recommended that patients with mild LUTS or symptoms that are not bothersome be administered any form of treatment. Instead, patients’ symptoms are monitored conservatively with a urologist or primary care physician (watchful waiting), and lifestyle modifications that can help control the severity of a patient’s LUTS are implemented. (5, 9) These lifestyle modifications include: avoiding caffeinated beverages, alcohol, and spicy foods; restricting fluids (especially before bedtime); implementing pelvic floor exercises; preventing any form of constipation; and avoiding medication that have effects on LUTS such as diuretics, decongestants, antihistamines, and antidepressants. (5) Medications or surgery are suggested if the symptoms become more bothersome. (5, 9, 18)
Society guidelines recommend that patients with moderate LUTS be offered pharmacotherapy as part of their treatment. (5) However, if these medications do not help improve LUTS and/or cause undesired side effects, urologists suggest stopping these medications and selecting a new therapy instead. The alternative therapy can be in the form of either a new medical or a surgical approach.
The selection of medications for the management of BPH relies on symptom severity, comorbidities, and side effect profile because all recommended medications have equal clinical effectiveness. (5)
Although all recommended medications for BPH have equal clinical effectiveness, alpha-1-adrenergic antagonists (alpha blockers) are strongly recommended as first-line therapy. (5) Additional medical therapies include phosphodiesterase-5 inhibitors (PDE5I), 5-alpha-reductase inhibitors (5-ARI), or a combination of these two with alpha blockers.
To treat males with severe symptoms, patients can either opt for medical or surgical therapy. Medical therapies are usually prescribed as combination therapies (i.e. alpha-1-adrenergic antagonist + 5-ARIs). Surgery is recommended for patients experiencing LUTS that are secondary to BPH when voiding symptoms are severe, watchful waiting and treatment with medications have been unsuccessful, or if the patient has a preference. (19)
As seen in Flowchart 1, certain clinical presentations call for an alteration to the course of a proposed treatment, regardless of the patient’s IPSS score. For instance, if the patient experiences urinary retention, recurrent UTIs, recurrent/persistent gross hematuria, bladder stones, or renal insufficiency, then immediate surgical treatment is indicated. (9)
The number of surgical modalities available to treat LUTS, secondary to BPH, is growing. These options include monopolar transurethral resection of the prostate (TURP), bipolar TURP, greenlight laser photovaporization, enucleation, Rezum, Urolift, Aquablation, open simple prostatectomy, and robotic simple prostatectomy. Before proceeding with surgery, the prostatic volume must be accurately measured with either a transrectal or transabdominal ultrasound, as the availability of these treatments relies heavily on the patient’s prostatic volume. (5)
The available surgical modalities for BPH vary in degree of invasiveness, risk of complications, functional outcomes, effects on patient’s health-related quality of life, and cost. (3, 20) The variation within these treatments allow patients to select an option that best meets their personal preferences. For example, novel treatments are available to provide better sexual outcomes in the case of postoperative ejaculatory dysfunction—specifically retrograde ejaculation which is a common complication secondary to most surgical therapies. (21) Therefore, implementing a patient-centered approach that emphasizes shared decision making is essential before recommending a treatment for a patient’s LUTS secondary to BPH. (22)
To conclude, BPH is a condition that can lead to LUTS which, in turn, can have a significant negative impact on patients’ HRQoL. Importantly, the clinician must recognize that LUTS possess many different aetiologies. Therefore, a methodical stepwise approach like the one described should be carefully followed. Currently, many options are available to manage LUTS either medically or surgically. These treatments present their own risks and benefits. As such, a shared decision-making process should be implemented.
Beyond the initial approach
Alpha-1-adrenergic antagonists (alpha blockers)
Alpha blockers relax the smooth muscle within the prostatic parenchyma and the bladder neck, thus facilitating urination by decreasing luminal resistance. The main advantage of this medication is relatively rapid symptom relief. The main disadvantages of this medication are hypotension and ejaculatory dysfunction. (23) Examples of common alpha-1-adrenergic antagonists are tamsulosin, alfuzosin, and silodosin. (5)
5-alpha-reductase inhibitors (5-ARI)
If patients experience side-effects such as hypotension but still desire medical therapy, they can be switched to 5-alpha-reductase inhibitors (5-ARI). 5-ARIs block the conversion of testosterone to dihydrotestosterone, which is the main molecular signal for prostatic growth. The main disadvantages of this medication are decreased libido, erectile dysfunction, and ejaculatory dysfunction. (23) Examples of common 5-ARIs include finasteride and dutasteride. (5)
Phosphodiesterase-5 inhibitors (PDE5I)
PDE5Is relax the prostatic tissue and the bladder neck making it easier to urinate. This medication is also used to treat erectile dysfunction. (23) So, when patients experience erectile dysfunction and LUTS secondary to BPH, these medications are ideal to treat both issues simultaneously. The main disadvantages of this medication are headaches and stomach aches. (23) Additionally, PDE5I are contraindicated in patients taking organic nitrates in any form, as the combination of both medications can lead to severe hypotension. An example of a commonly used PDE5I is tadalafil. (5)
References
- Vuichoud C, Loughlin KR (2015) Benign prostatic hyperplasia: epidemiology, economics and evaluation. Can J Urol 22:1-6.
- Yeboah E (2016) Prevalence of Benign Prostatic Hyperplasia and Prostate Cancer in Africans and Africans in the Diaspora. Journal of the West African College of Surgeons 6:1.
- Erkoc M, Otunctemur A, Besiroglu H, et al. (2018) Evaluation of quality of life in patients undergoing surgery for benign prostatic hyperplasia. Aging Male 21:238-42.
- Wei JT, Calhoun E, Jacobsen SJ (2005) Urologic diseases in America project: benign prostatic hyperplasia. The Journal of urology 173:1256-61.
- Nickel JC, Aaron L, Barkin J, et al. (2018) Canadian Urological Association guideline on male lower urinary tract symptoms/benign prostatic hyperplasia (MLUTS/BPH): 2018 update. Can Urol Assoc J 12:303.
- McVary KT. Clinical manifestations and diagnostic evaluation of benign prostatic hyperplasia. Waltham, MA: UpToDate; 2020.
- Badalato G, Amiel G, Hollis M, et al. Medical Student Curriculum: Benign Prostatic Hypertrophy (BPH) 2020 [cited 2020 19 December]. https://www.auanet.org/education/auauniversity/for-medical-students/medical-students-curriculum/medical-student-curriculum/bph.2020 19 December, 2020.
- Barry MJ, Fowler FJ, O'leary MP, et al. (2017) The American Urological Association symptom index for benign prostatic hyperplasia. The Journal of urology 197:S189-S97.
- McVary KT, Roehrborn CG, Avins AL, et al. (2011) Update on AUA guideline on the management of benign prostatic hyperplasia. The Journal of urology 185:1793-803.
- Abrams P, Chapple C, Khoury S, et al. (2009) Evaluation and treatment of lower urinary tract symptoms in older men. The Journal of urology 181:1779-87.
- Wuerstle MC, Van Den Eeden SK, Poon KT, et al. (2011) Contribution of common medications to lower urinary tract symptoms in men. Archives of internal medicine 171:1680-2.
- Rendon RA, Mason RJ, Marzouk K, et al. (2017) Canadian Urological Association recommendations on prostate cancer screening and early diagnosis. Canadian Urological Association Journal 11:298.
- Madersbacher S, Alivizatos G, Nordling J, et al. (2004) EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines). European urology 46:547-54.
- Nguyen DD, Trinh QD, Cole AP, et al. (2021) Impact of health literacy on shared decision making for prostate-specific antigen screening in the United States. Cancer 127:249-56.
- Carter HB, Albertsen PC, Barry MJ, et al. (2013) Early detection of prostate cancer: AUA Guideline. The Journal of urology 190:419-26.
- Alawamlh OAH, Goueli R, Lee RK (2018) Lower urinary tract symptoms, benign prostatic hyperplasia, and urinary retention. Medical Clinics 102:301-11.
- D’Silva KA, Dahm P, Wong CL (2014) Does this man with lower urinary tract symptoms have bladder outlet obstruction?: The Rational Clinical Examination: a systematic review. Jama 312:535-42.
- Foster HE, Barry MJ, Dahm P, et al. (2018) Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. The Journal of urology 200:612-9.
- NICE. Quality Standard 45: Lower urinary tract symptoms in men [cited 2020 October 12]. www.nice.org.uk/guidance/QS45 October 12, 2020.
- Shi-Wei H, Chung-You T, Chi-Shin T, et al. (2019) Comparative efficacy and safety of new surgical treatments for benign prostatic hyperplasia: systematic review and network meta-analysis. BMJ: British Medical Journal (Online) 367.
- Sadri I, Arezki A, Couture F, et al. (2020) Reasons to overthrow TURP: bring on Aquablation. World journal of urology:1-9.
- Bouhadana D, Nguyen D-D, Schwarcz J, et al. (2021) Development of a patient decision aid for the surgical management of lower urinary tract symptoms secondary to benign prostatic hyperplasia. BJU international 127:131-5.
- Yu Z-J, Yan H-L, Xu F-H, et al. (2020) Efficacy and Side Effects of Drugs Commonly Used for the Treatment of Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia. Frontiers in Pharmacology 11:658.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.