Approach to
Amenorrhea
Sophie Baril1
Published online: 12 April 2021
1McGill University, Montreal, QC, Canada
Corresponding Author: Sophie Baril, email sophie.baril@mail.mcgill.ca
DOI: 10.26443/mjm.v19i1.320
Abstract
This article provides an approach to amenorrhea and is intended for pre-clinical and clerkship medical students. Primary amenorrhea refers to the absence of menarche by 15 years or 3 years post thelarche while secondary amenorrhea is the cessation of menses for 3 months in women with a previously regular cycle or for 6 months in women with previously irregular menses. While amenorrhea can be physiological it can also reflect an anatomical or more complex hormonal problem that students must learn to identify and investigate.
Tags: Anatomical defects, Hypogonadism, Primary amenorrhea, Secondary amenorrhea
Question
A 15-year-old girl presents to your office. Her primary concern is that she is shorter than her classmates. She mentions she has had hearing difficulties since childhood, and is known for a heart defect and scoliosis. She is concerned about being the only one in her not having had her period yet. On physical exam, the patient is short in stature with no physical signs of pubertal development. You also notice that she has low set ears and a low hairline. Initial laboratory results are as follows:
Beta Human Chorionic Gonadotropin (hCG): 1mIU/mL (normal value in non-pregnant women <5mIU/mL)
Follicle-stimulating Hormone (FSH): 50mIU/mL (normal value between 5-20mIU/mL)
Luteinizing Hormone (LH): 48mIU/mL (normal value between 5-20mIU/mL)
Prolactin (PRL): 27µg/L (normal value in non-pregnant women 2-29µg/L)
Which of the following would not be indicated as the next step in the diagnosis of this patient's amenorrhea?
- Karyotype
- Thyroid function tests
- Pelvic ultrasound (US)
- Testosterone and 17-OHP levels
Answer
D) The patient's presentation is consistent with primary amenorrhea, most likely due to Turner Syndrome (TS). The elevated FSH and LH levels are representative of hypergonadotropic hypogonadism, most likely caused by gonadal dysgenesis in this case(1). The physical appearance of this patient as well as the implication of other organ systems are also suggestive of TS, but the diagnosis can only be confirmed by karyotype. Many patients with TS also suffer from hypothyroidism which should be investigated when detected on laboratory testing for amenorrhea (1). In this case, the amenorrhea is caused by the gonadal dysgenesis and not the hypothyroidism per se. Pelvic US should always be included in the initial investigation of primary amenorrhea to confirm the presence of a uterus and vagina. In TS, ovaries will appear as fibrous streaks without any follicles (1). Testosterone and 17-OHP testing are not useful in the initial diagnosis of TS and would be more relevant if the patient had signs of hyperandrogenism or high LH/FSH ratio to rule out malignancy, congenital adrenal hyperplasia or polycystic ovary syndrome (PCOS). Note that TS can be characterized by monosomy or mosaicism and that the manifestations of this condition can vary dramatically from one person to another.
Main Text
Initial Approach
The initial investigation of a patient presenting with amenorrhea begins with a thorough history and physical examination. First, it is important to differentiate between primary and secondary amenorrhea. (2) The former refers to the absence of menarche by 15 years or 3 years post-thelarche, while the latter is the cessation of menses for 3 months in women with a previously regular cycle. (3) For primary amenorrhea, a complete growth history as well inquiry as to the onset and progression of puberty is crucial. In addition to the history of present illness, a review of the patient's previous menstrual patterns, gynecologic instrumentation and general health status are important to ask for on history when secondary amenorrhea is suspected. (4, 5) On physical examination, the clinician should look for the presence of secondary sexual characteristics, signs of virilization, presence of dysmorphic features, and signs of thyroid disease. (4, 5) Abdominal and pelvic examinations should also be performed. While investigating the cause of amenorrhea may be necessary, it is important to keep in mind that constitutionally late menarche or early menopause can also explain these clinical manifestations.
Beta Human Chorionic Gonadotropin
The first step in the evaluation of amenorrhea is to exclude pregnancy because it is the most common cause of amenorrhea in women of reproductive age. (5) Measurement of serum or urine hCG levels are the most common laboratory tests ordered to evaluate pregnancy. hCG is produced by syncytiotrophoblast cells and rises exponentially during the first trimester of pregnancy. (5) Serum testing is very sensitive and can detect hCG amounts as low as 1-2mIU/mL. (6) Normally, hCG levels are insignificant in non-pregnant women but high levels can occur in the presence of malignancies producing very high levels of ectopic hCG such as choriocarcinoma, germ cell tumors and hydatidiform moles. (5, 6) If the hCG laboratory testing is positive, US should be performed next to evaluate the presence of an intrauterine or ectopic pregnancy. (2)
Prolactin
Hyperprolactinemia is the most common cause of secondary amenorrhea after pregnancy and is diagnosed when serum levels of PRL exceed 25µg/L. (7, 8) Elevated PRL disrupts gonadotropin-releasing hormone (GnRH) pulsatile secretion which results in hypogonadotropic hypogonadism (HH). PRL levels of 20-200µg/L are often due to the use of prescription drugs or lactotroph microadenomas, while a PRL level of more than 200µg/L is mostly caused by a macroprolactinoma. (7, 8) The presentation of a prolactinoma depends on its size; ranging from symptoms of hyperprolactinemia such amenorrhea, loss of libido and galactorrhea to mass effect occurring with macroprolactinomas. (7) If hyperprolactinemia cannot be explained by the use of medications, a physiologic cause or dysfunction of another organ system, an MRI should be ordered to investigate the possibility of a pituitary gland tumor. (7, 8)
Thyroid Stimulating Hormone
Menstrual irregularities are frequent consequences of thyroid dysfunction. (9) Amenorrhea can be caused by hypothyroidism or hyperthyroidism. Once pregnancy has been ruled out, thyroid stimulating hormone (TSH) level should be measured as the initial investigation of a thyroid disorder. TSH is low in hyperthyroidism, which leads to an excess of sex hormone-binding globulin (SHBG). (9, 10) The result is a reduction of the free fraction of estrogen and testosterone available in the circulation, causing anovulation and amenorrhea. (9, 10) Amenorrhea can also be a symptom of hypothyroidism, which also causes a reduction in serum SHBG, resulting in a decrease in total circulating estrogen and testosterone. (9, 10) Ovulatory dysfunction may also be caused by hyperprolactinemia from long-standing increases in thyrotropin-releasing hormone levels seen in hypothyroidism. (9, 10) The underlying cause of thyroid dysfunction should be investigated further if abnormal TSH levels are found on initial investigation of amenorrhea.
Pelvic Ultrasound
Anatomical defects should always be considered in the differential diagnosis of primary amenorrhea. Women presenting with primary amenorrhea should be evaluated for the presence of a uterus and vagina by pelvic US. (3, 4) Observation of a shortened vaginal canal or absent uterus should prompt investigation for Müllerian agenesis and androgen insensitivity syndrome (AIS). (3, 11) These two conditions can be differentiated by karyotyping or measuring serum testosterone (Figure 1, Table 1). Outflow tract obstructions such as an imperforate hymen or a transverse vaginal septum often present with cyclic pain and may be the cause of amenorrhea in the presence of a normal uterus. (3, 11) Pelvic US is also relevant in the investigation of secondary amenorrhea to assess for ovarian pathology as well as PCOS. Intrauterine adhesions and cervical stenosis resulting from endometrial instrumentation and cervical procedures also need to be considered in the differential diagnosis of secondary amenorrhea. (3, 11)
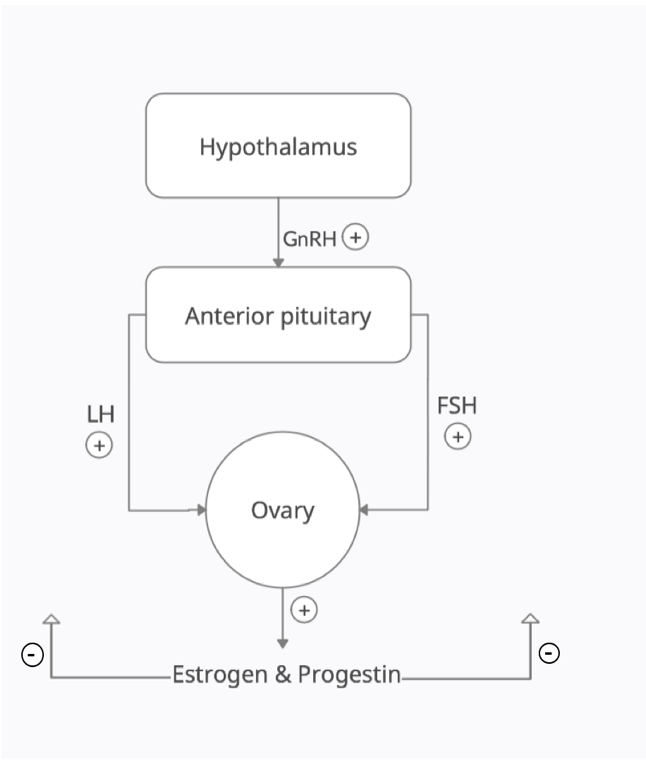
Depending on the level of dysfunction within the HPO axis, the serum levels of FSH and LH will be different. When the defect is downstream at the level of the ovaries, FSH and LH levels will be elevated in the absence of negative feedback from the gonads (hypergonadotropic hypogonadism). When the defect is more upstream (hypothalamus or pituitary), the patient will suffer from HH and the serum levels of FSH and LH will be low.
| Table 1 | Type of amenorrhea | FSH | LH | LH/FSH | PRL | Testosterone | 17-OHP |
| Hypergonadotropic Hypogonadism | Ovarian Failure
Gonadal dysgenesis Premature ovarian insufficiency |
↑ | ↓ | ↔ | ↔ or ↓ | ↔ | |
| Normogonadotropic Hypogonadism | Outflow tract obstruction | ↔ | ↔ | ↔ | ↔ | ↔ | |
| PCOS | ↑ | ↑ | ↑ | ↔ | |||
| Adrenal or ovarian malignancy | ↔ | ↔ | ↑↑ | ↔ | |||
| Late-onset congenital adrenal hyperplasia | ↑ | ↔ | ↑ | ↑ | |||
| Hypogonadotropic Hypogonadism | Hyperprolactinemia | ↓ | ↑ | ↑ | ↔ | ↔ | |
| Hypothalamic amenorrhea | ↔ | ↓ | ↔ or ↓ | ↔ | |||
↔: Normal levels ↑: High levels ↑↑: Very high levels ↓: Low levels
Adapted from: Amenorrhea: A systematic Approach to Diagnosis and Management, AAFP. https://www-aafp-org.proxy3.library.mcgill.ca/afp/2019/0701/afp20190701p39.pdf
Follicle-stimulating Hormone, Luteinizing Hormone and Estradiol
Independent of the classification of the patient's amenorrhea as primary or secondary, the remaining causes can be divided into normogonadotropic, hypergonadotropic or HH, depending on FSH and LH serum values (Table 1). FSH, LH and estradiol levels will be increased or decreased depending on which component of the hypophyseal-pituitary-ovarian (HPO) axis is dysfunctional (Figure 2). Normal values of FSH and LH are between 5-20mIU/mL, but LH levels can increase up to 40mIU/mL during the LH surge 24h prior to ovulation (11, 12). Normal values of estradiol vary between 30-400pg/mL in premenopausal women and 0-30pg/mL in postmenopausal women. Elevated levels of FSH and LH with low estradiol are characteristic of ovarian failure, while low levels of these hormones are a feature of hypothalamic or pituitary dysfunction. (11, 12) It is also important to note that pregnant women or women taking exogenous estrogens would have high estradiol with low FSH and LH. Specific causes of each category are discussed below, and further management depends on the diagnosis. When there is evidence of hyperandrogenism, PCOS should be considered as well as the possibility of malignancy and late-onset congenital adrenal hyperplasia (CAH). (13, 14)
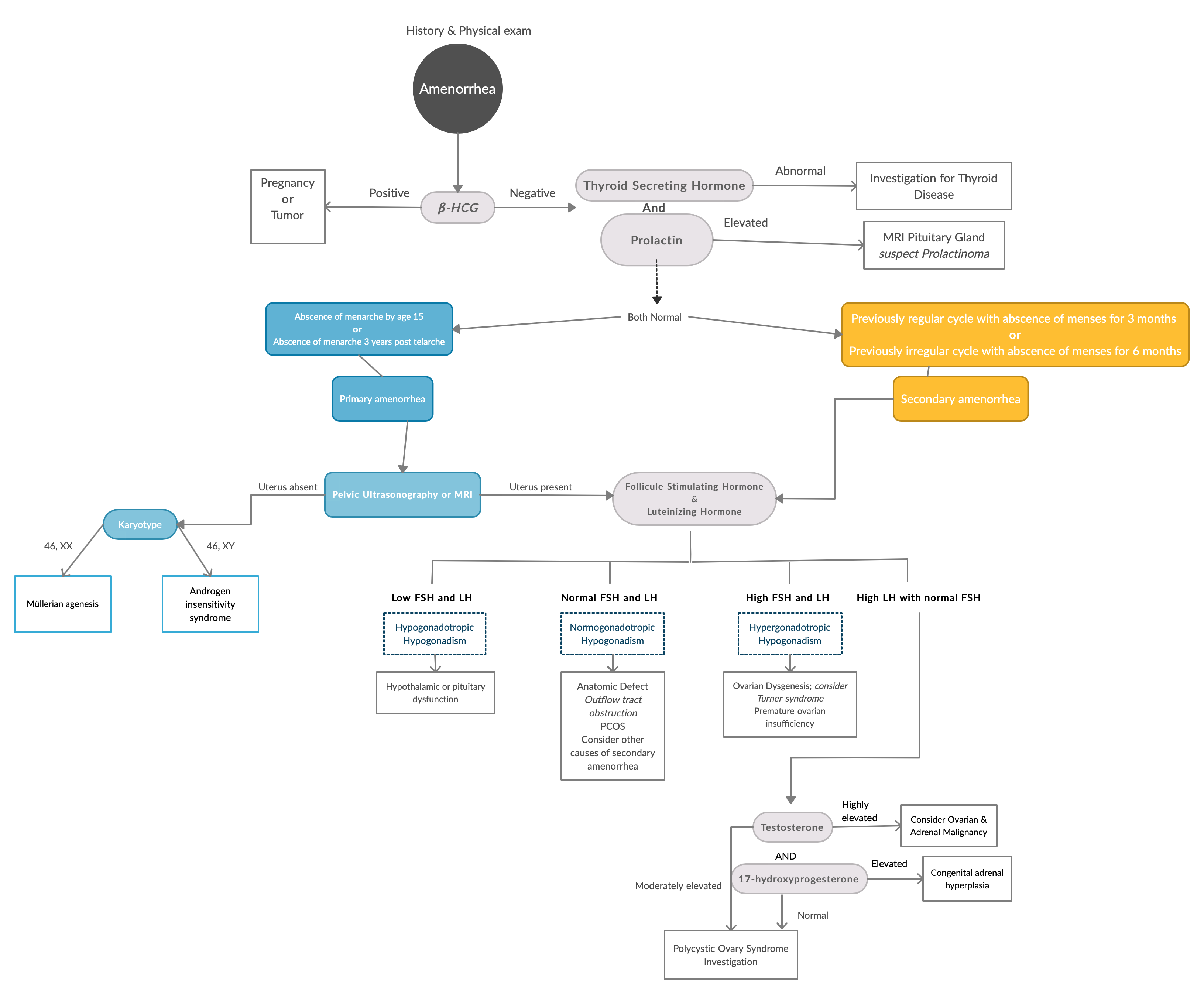
Using an algorithmic approach to secondary amenorrhea: Avoiding diagnostic error, Clinica Chimica Acta. https://pdf.sciencedirectassets.com/271330/1-s2.0-S0009898113X00075/1-s2.0-S0009898113001411/main.pdf
Systematic approach to the evaluation of amenorrhea. The differential diagnosis of amenorrhea can be divided into two broad categories; primary and secondary amenorrhea. It is important to note that etiologies of secondary amenorrhea can also be the cause of primary amenorrhea. If the patient has a uterus, the evaluation of primary and secondary amenorrhea is similar following history and physical examination. Note that in real life, FSH and LH levels are often measured with TSH and PRL not to delay diagnosis.
Beyond the Initial Approach
In this section, important causes of amenorrhea are discussed in more detail, including further steps in patient management and treatment.
Polycystic Ovary Syndrome
PCOS is the most common cause of infertility in women and is a major cause of hyperandrogenic amenorrhea. (13) It should be considered in every patient presenting with amenorrhea and signs of virilization, such as hirsutism and acne. Diagnosis of PCOS requires two out of three of the following criteria: clinical or laboratory evidence of hyperandrogenism, oligo or amenorrhea, and polycystic ovaries on US. (13) Other causes of hyperandrogenism and menstrual irregularities must also be excluded to make a diagnosis of PCOS. These include androgen secreting tumors and CAH. Often, the criteria for hyperandrogenism are met on physical examination but biochemical measurement of androgens should be included in the initial investigation as it helps differentiate PCOS from an androgen secreting tumor (13). The first step in the management of patients with PCOS is diet and exercise. Given that PCOS is associated with insulin resistance, metabolic screening is also recommended and treatment with metformin may be beneficial. Finally, since chronic anovulation is associated with an increased endometrial cancer risk, patients can be managed with cyclic progesterone every 3 months if there is no spontaneous menses or with the use of combined oral contraceptives to ensure regular menstrual bleeding if pregnancy is not being pursued. (13)
Anatomical defect: Müllerian agenesis vs Androgen Insensitivity Syndrome
Müllerian agenesis and AIS both cause primary amenorrhea. Patients with either pathologies will have an absent or hypoplastic uterus and upper vagina which can be initially observed on US. (14, 15) It is important to distinguish the two entities by karyotype to direct management. Patients with Müllerian agenesis have a 46XX karyotype and the HPO axis remains unaffected. (15) The ovaries are functional, and the patient will have female secondary sexual characteristics. Patients with AIS have a 46XY karyotype but have a defect in the androgen receptor preventing external male genitalia development. (14, 15) Excess aromatization of their male-levels of androgens to estrogen allows for the development of female secondary sexual characteristics. (14) Management of both pathologies should address functional, sexual and psychologic issues. (14, 15) Gonadectomy after puberty is recommended for patients with AIS because of their high risk of cancer when gonads are left in the abdominal cavity. (14, 15) Other anatomical causes of primary amenorrhea that can be corrected surgically include imperforate hymen and vaginal septum.
Hypergonadotropic hypogonadism: Turner Syndrome and Premature Ovarian Insufficiency
TS can be found in about 1/3 of patients with gonadal dysgenesis. (1) It should be suspected in women presenting with primary amenorrhea and hypergonadotropic hypogonadism and can be confirmed by karyotyping (usually 45X0 or mosaic). (1, 14) In TS, the ovaries degenerate during fetal life or early childhood, explaining the decreased level of estradiol and the absence of pubertal development. (1) Considering the involvement of many organ systems and the possibility for complications, patients should be managed by a multidisciplinary team and hormone replacement therapy is usually recommended. (1) Premature ovarian insufficiency can also be the cause of secondary amenorrhea. While chemotherapy in young children can lead to primary amenorrhea, autoimmune diseases or iatrogenic causes such as surgery, chemotherapy and radiation are often the culprit for acquired ovarian failure occurring later in life. (11) The diagnosis is often suspected on history and management depends on the cause.
Hypogonadotropic hypogonadism: Functional hypothalamic amenorrhea
HH affects upstream levels of the HPO axis, either the hypothalamus or the pituitary gland (Figure 2). Another common cause of secondary amenorrhea is functional hypothalamic amenorrhea (FHA). (9, 12) FHA is characterized by dysfunction in the pulsatile release of GnRH by the hypothalamus, resulting in abnormal levels of FSH and LH and reduction of estradiol production by the ovaries. (9, 12) FHA can often be confused with pituitary dysfunction as a cause of HH but will respond to a GnRH stimulation test unlike the latter. (12) Major causes of hypothalamic disturbances include stress, weight loss and exercise, however organic causes should also be ruled out by imaging. (12) Particular attention should be placed on the presence of underlying anxiety or mood disorders and a bone density scan may be indicated for women with amenorrhea lasting for more than 6 months. (12) Amenorrhea is usually reversible, and treatment depends on the cause but mostly relies on adequate nutritional status, stress reduction and treatment of underlying psychiatric disorders.
References
- Morgan T. Turner Syndrome: Diagnosis and Management. American Family Physician. 2007:76(3): 405-410.
- Practice Committee of American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril. 2008;90(5):219-25.
- Klein DA, Paradise SL, Reeder RM. Amenorrhea: A Systematic Approach to Diagnosis and Management. Am Fam Physician. 2019 Jul 1;100(1):39-48. PMID: 31259490.
- Heiman DL. Amenorrhea. Prim Care. 2009 Mar;36(1):1-17.
- Roberts-Wilson TK, Spencer JB, Fantz CR. Using an algorithmic approach to secondary amenorrhea: Avoiding diagnostic error. Clin Chim Acta. 2013 Aug 23;423:56-61.
- Betz D, Fane K. Human Chorionic Gonadotropin (HCG) [book on the Internet]. Treasure Island (FL): StatPearls; 2020 [Updated 2020 Aug 30; cited Sept.24 2020]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532950/
- Glezer A, Bronstein MD. Prolactinomas. Endocrinol Metab Clin North Am. 2015;44(1):71-78.
- Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci. 2013;6(3):168-175.
- Kalro BN. Impaired fertility caused by endocrine dysfunction in women. Endocrinol Metab Clin North Am. 2003;32(3):573-92.
- Kravets I. Hyperthyroidism : Diagnosis and Treatment. American Family Physician. 2016:93(5):363-373.
- Jankowska K. Premature ovarian failure. Prz Menopauzalny. 2017;16(2):51-56.
- Meczekalski B, Katulski K, Czyzyk A, Podfigurna-Stopa A, Maciejewska-Jeske M. Functional hypothalamic amenorrhea and its influence on women's health. J Endocrinol Invest. 2014;37(11):1049-1056.
- Legro R S, Arslanian S A, Ehrmann D A, Kathleen M. Hoeger, Murad H M, Pasquali R, Welt C K. Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism. 2013: 98 (12):4565-4592.
- Yoon JY, Cheon CK. Evaluation and management of amenorrhea related to congenital sex hormonal disorders. Ann Pediatr Endocrinol Metab. 2019;24(3):149-157.
- Committee on Adolescent Health Care. Müllerian Agenesis: Diagnosis, Management, and Treatment. Obstetricts & Gynecology. 2018;131(1):35-42.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.