Approach to
Chest pain
Nasim Haghandish1
Published online: 10 April 2021
1McGill University, Montreal, QC, Canada
Corresponding Author: Nasim Haghandish, email nasim.haghandish@mail.mcgill.ca
DOI: 10.26443/mjm.v19i1.314
Abstract
The diagnosis of chest pain is not always due to a cardiac cause such as a myocardial infarction. In fact, non-cardiac causes of chest pain may present with similar signs and symptoms. This article delves into the differential diagnosis of chest pain using an anatomical approach and describes how a thorough history, physical examination as well as specific tests are required to confirm each diagnosis.
Tags: Acute chest pain, Anatomical approach, Diagnosis
Question
An overweight 55-year-old man presents to the emergency department with sudden, sharp, and severe interscapular chest pain. The pain began about 30 minutes ago and the patient also reports weakness, diaphoresis, and dyspnea. The patient has Marfan syndrome and has had hypertension for the past 10 years, for which he is taking telmisartan. He does not smoke. On physical exam, there is an increased respiration rate and pulse of 88 beats per minute (bpm) that was weaker in his right arm. Blood pressure of both arms were the following: 165/80 (left) and 120/70 (right). Electrocardiogram revealed ST-segment elevation in leads II, III, and aVF. What is the next best step?
Which condition is most consistent with the clinical presentation?
- Exercise stress test
- Thrombolytic therapy
- CT angiogram
- Transthoracic echocardiogram
- Percutaneous coronary intervention
Answer
C. The patient presents with an aortic dissection that needs to be urgently diagnosed before further steps are taken. The sudden, sharp, ripping/tearing pain radiating to the back, accompanied with diaphoresis, pulse and blood pressure asymmetry, and weakness are typical signs noted in aortic dissection. Additionally, Marfan syndrome is a common risk factor, as is hypertension (the most common risk factor). In fact, an acute rise in blood pressure may cause an aortic dissection, specifically in the case of cocaine drug use. Other physical exam findings include aortic regurgitation and focal neurological deficits. If there is compression on other structures such as the superior cervical ganglion, superior vena cava, or esophagus, patients may also present with Horner’s syndrome, superior vena cava syndrome, and dysphagia, respectively. Furthermore, extension of the dissection flap may cause obstruction of the branching vessels off the aorta, increasing the risk of complications and death.
In this case, the next best step is to diagnose an aortic dissection then follow up with management. A CT angiogram is the gold standard for the diagnosis of aortic dissection and is useful in the pre-operative planning to potentially rule out distal arterial involvement such as renal artery dissections. Following diagnosis, the blood pressure should be reduced with beta-blockers or nitroprusside to reduce shear forces on the aortic wall/intima. Depending on the type of aortic dissection (Stanford classification), management differs. Type A (involving the ascending aorta and the arch) is treated surgically whereas type B (involving the descending aorta) is generally managed medically or endovascularly.
Aortic dissections can develop with a concomitant myocardial infarct, specifically occlusion of the right coronary artery (RCA) resulting in an ST-segment elevation pattern in the inferior leads. (1) A misdiagnosis can result in fatality as treatment with a thrombolytic or anticoagulant could result in severe bleeding.
Initial Approach
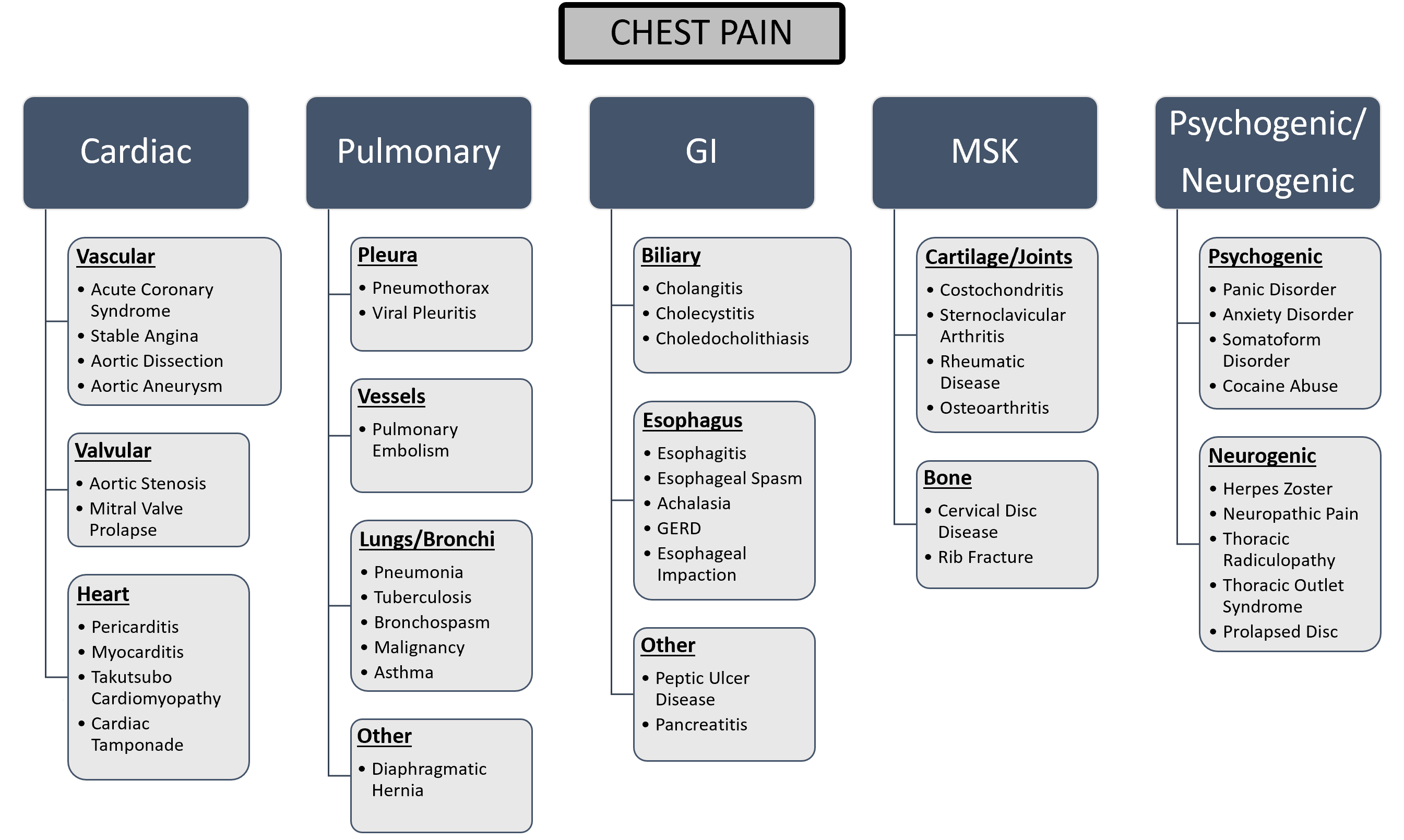
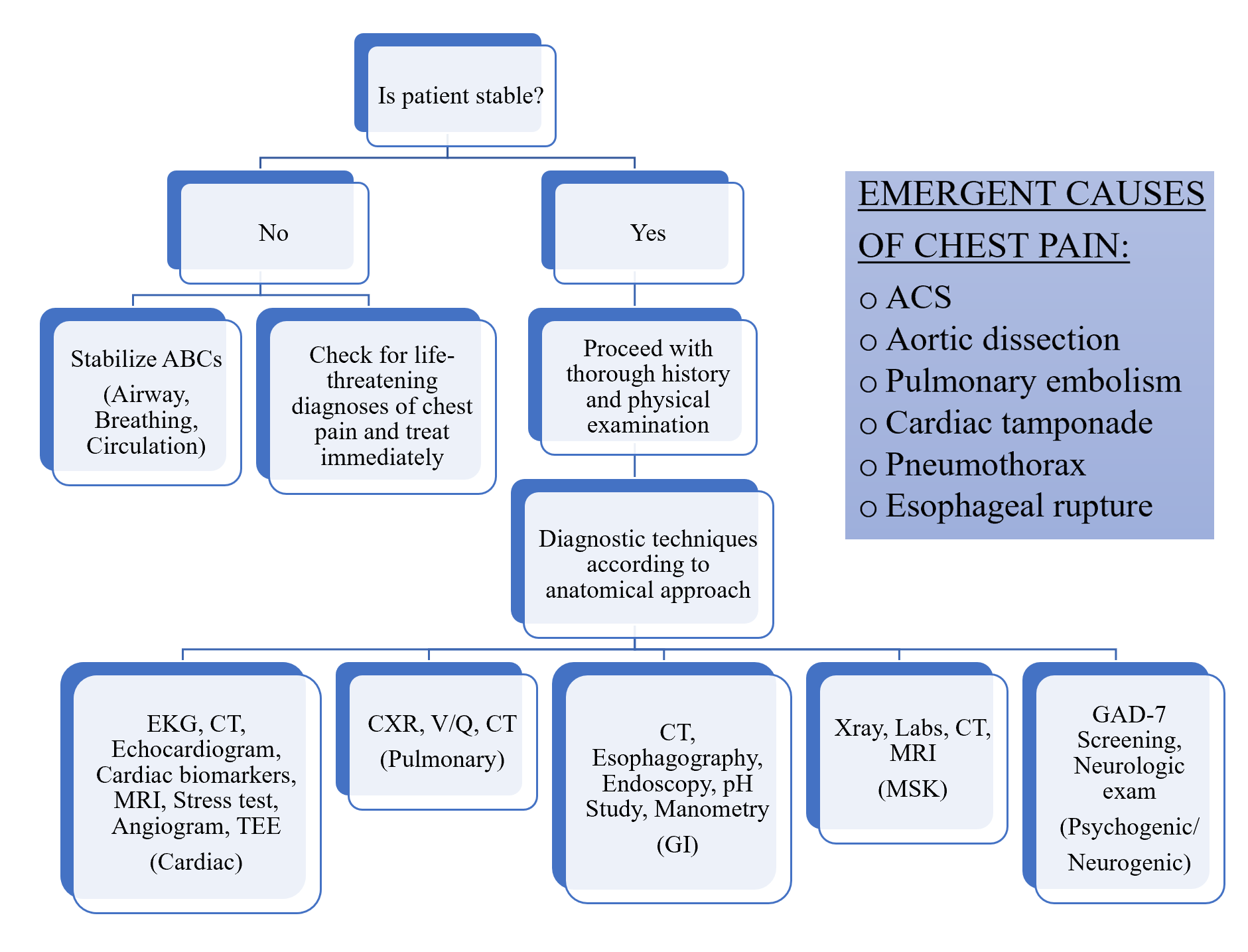
Chest pain can be divided into cardiac vs non-cardiac origin. Non-cardiac chest pain can be further subdivided using an anatomical approach: gastrointestinal (GI), pulmonary, musculoskeletal (MSK), and psychogenic/neurogenic pain (Figure 1). (2–5) When evaluating chest pain, it is pertinent to first stabilize the patient and rule out any life-threatening conditions that require immediate treatment such as an acute coronary syndrome (ACS), aortic dissection, pericarditis/cardiac tamponade, pulmonary embolism, pneumothorax, and esophageal rupture (Flowchart 1).
History
For an adequate medical history, the following information should be gathered: onset (abrupt or gradual), palliation/provocation, quality (sharp, dull, squeezing, pleuritic, tightness, ripping), radiation, intensity, positioning, and timing of the pain (recurrent/intermittent, new). With this information, one can begin to use the anatomical approach to identify the cause of the chest pain.
Cardiac chest pain is a visceral type of pain; hence it is poorly localized. Patients complain of squeezing, aching, dullness, pressure, tightness, ripping, and burning pain that tends to radiate to the arms or jaw. (2–5) An ACS can be categorized as either an ST-segment elevation myocardial infarct (STEMI), non-ST-segment elevation myocardial infarct (NSTEMI), or unstable angina. Importantly, the retrosternal chest pain from ACS typically lasts longer than 30 minutes, is exacerbated by activity, and is relieved by rest/nitroglycerin. ACS symptoms described by women, elderly, and diabetic patients may differ slightly from those listed above as there can be atypical symptoms such as diaphoresis, dyspnea, epigastric pain, back pain, nausea and vomiting, unusual fatigue, and indigestion. (6) For chest pain that a physician deems appropriate for ACS workup, the HEART score can be used to predict the risk of major cardiac events over 6 weeks in order to facilitate diagnosis and therapeutic choices for a patient displaying cardiac chest pain. Moreover, the T-MACS decision aid is helpful in ruling out an ACS using more regimented descriptors which can ultimately help medical learners to differentiate between high, medium, and low suspicion of ACS. In the event of an unstable angina or NSTEMI, the TIMI/GRACE score may be used to estimate patient’s mortality and can correlate with the risk of adverse outcomes as well as to guide early invasive vs medical management.
Another example of cardiac pain is an aortic dissection, which presents with a ripping, intense pain that radiates to the back. An ADD-RS score and D-Dimer are useful in ruling out an aortic dissection in low to moderate-risk patients. Pericarditis is unique in that it presents with substernal pain with concomitant pleuritic pain. Moreover, the pain is usually positional (relieved when leaning forward). In pericarditis, the substernal pain often radiates to the shoulder and can sometimes be mistaken for ACS.
GI pain is visceral and tends to be related to food intake and recumbency, and can often be relieved by antacids. However, certain diagnoses are difficult to differentiate from cardiac such as an esophageal spasm or rupture. (2,5,7,8) Nonetheless, it is better to rule out an ACS by performing diagnostic investigations than to misdiagnose. Interestingly, studies have shown that patients with gastroesophageal reflux disease (GERD) are at a higher risk of developing an acute myocardial infarct (MI); thus, GERD diagnoses do not necessarily rule out an ACS but in fact may increase the likelihood of an ACS event. (9,10) Upper GI pathologies such as cholecystitis and pancreatitis may also exhibit some pulmonary-type symptoms as diaphragmatic irritation may occur. Esophageal rupture is a life-threatening cause of chest pain that is most commonly caused by increased intra-esophageal pressure (such as forceful vomiting) leading to a barogenic esophageal rupture. The chest pain in esophageal rupture is typically associated with vomiting and subcutaneous emphysema, termed Mackler triad. (11)
Pulmonary pain is described as pleuritic, meaning that the pain is enhanced or sharpened on inspiration or following a cough. A pneumothorax has an acute onset of pleuritic pain with dyspnea. (2,8) Pulmonary embolism (PE) typically presents with dyspnea, chest pain, cough, and fever and if suspected, the risk and pre-test probability can be estimated using Well’s criteria. The PERC rule can be additionally used to rule out PE in patients who are deemed low risk with a pre-test probability <15% to ultimately avoid any further unnecessary testing. Depending on the scores, further testing may be required such as a D-dimer or CT angiogram to rule out a PE.
MSK pain is a somatic pain rather than visceral and tends to be darting, sharp, very well localized, positional, and worsens with movement; the pain can be reproduced upon palpation, specific movements, and positions. Some examples include rheumatic disease, costochondritis, fibromyalgia, and fractures secondary to trauma. (8)
Psychogenic pain such as panic attack is much harder to diagnose as the symptoms may overlap with the other subtypes. However, a good psychosocial history and GAD-7 screening is helpful in the primary care setting. (8,12) Neurogenic pain is typically described as burning, tingling, electrical shock-like, stabbing pain that tends to follow dermatomes.
A history of past medical illness should be acquired, especially history of hypertension, diabetes, dyslipidemia, lung disease, and genetic disorders. Social history (specifically smoking, alcohol, and other illicit drugs including cocaine) and other associated symptoms such as fever, nausea, vomiting, syncope, dyspnea, and palpitations could additionally help narrow down the cause of the chest pain. A family history of cardiovascular disease, cardiomyopathy, and sudden or unexplained death are also pertinent in identifying potential genetic risk factors for concerning cardiac causes.
Physical Exam
Next, a systems approach for physical examination is employed, starting with vital signs. For instance, unequal pulse and pressure between both limbs suggest an aortic dissection and hypotension may indicate a vascular etiology, a pneumothorax, anxiety, or PE.
A targeted cardiac exam should identify heart sounds and murmurs: a fourth heart sound can indicate ischemia (due to impaired relaxation of the ventricle leading to acute diastolic dysfunction), a pericardial rub can indicate pericarditis, and a murmur is a typical finding of valvular disease – for instance, aortic regurgitation is particularly concerning for aortic dissection. Low blood pressure, muffled heart sounds, and jugular vein distension (also termed Beck’s triad) are indicative of cardiac tamponade and warrant immediate intervention. Volume status and leg edema should also be assessed to identify any cardiac etiology of chest pain such as volume overload indicating heart failure. Of note, jugular venous distension has a high negative predictive value for heart failure. Additionally, POCUS (Point-of-care ultrasound) is useful in the context of pericardial effusion.
The pulmonary exam should include auscultation of all lung lobes, listening for wheezes, rales, rhonchi, rubs, and bronchial breath sounds. Percussion of the lungs should assess for hyperresonance and decreased fremitus in the case of a pneumothorax or dullness if consolidation or pleural effusion is present. Rales and dullness may also suggest left-sided congestive heart failure. POCUS may also be used to rule out a pneumothorax if suspected: one must look for the absence of lung sliding and B lines, and the boundary/margin of the pneumothorax can be identified by the lung point.
An abdominal exam should also be performed to assess for abdominal tenderness, specifically in the right upper quadrant (suggesting liver or gallbladder pathologies). POCUS may also be used to better diagnose pathologies such as choledocholithiasis and cholecystitis; however, a good history and physical exam is more relevant. Furthermore, a formal ultrasound may be required prior to surgical management, if deemed necessary.
Finally, the Vancouver Chest Pain Rule may be used to identify patients who are low-risk and safe for an early discharge. This tool allows one to quickly discharge patients based on medical history, physical exam (especially reproducible palpable chest pain), electrocardiogram (EKG), and cardiac biomarkers.
Beyond the Initial Approach
In an academic hospital setting, 12-lead EKG and troponin levels are the first steps in diagnosing possible acute coronary syndrome. If pulmonary disease is suspected, a chest x-ray (CXR) can be ordered. (2,13,14)
When evaluating an EKG, one should be aware of the many different pathologies. Although not always the case, ischemia typically presents with an ST-segment depression whereas an ST-segment elevation indicates infarction or pericarditis. Furthermore, T wave abnormalities could also indicate ischemic disease or infarction. Signs of right heart strain, such as right axis deviation and right bundle branch block, could indicate pulmonary diseases such as PE. Electrical alternans and low EKG voltages suggests cardiac tamponade, which warrants pericardiocentesis or pericardiotomy as treatment modalities. (15)
The presence of cardiac biomarkers (particularly troponins) is helpful in differentiating active myocardial infarction from unstable angina. If valvular disease is suspected, an echocardiogram is helpful. Additionally, echocardiography is useful in diagnosing cardiac tamponade via cardiac chamber collapse, septal “bounce”, and inferior vena cava (IVC) dilatation. An MI can be identified by echocardiogram since regional wall motion abnormalities can be observed. (15,16) CT coronary angiogram can be useful for risk stratifying coronary artery disease which can subsequently be diagnosed via cardiac catheterization and treated with coronary stenting or coronary artery bypass grafting depending on disease severity and contraindications to therapies. A myocardial perfusion imaging test (MIBI) may also be used to identify areas of ischemia by comparing cardiac perfusion under rest and stressed conditions. (17) MIBI is extremely useful in identifying ischemic areas that can be salvaged by reperfusion.
For diagnosing life-threatening aortic dissection, a CT scan is utilized; alternatively, depending on the resources available a transesophageal echocardiography (TEE) is just as useful especially when a patient is hemodynamically unstable. Type A aortic dissections (which involve the ascending aorta and the arch; also the most common type) are typically repaired surgically whereas type B (involving the descending aorta) are managed medically with beta blockers and calcium channel blockers or endovascularly. (18) A CT scan may also be useful in diagnosing an MSK etiology secondary to a trauma. (5,8)
If a patient complains of pleuritic pain, a CXR is a rapid modality to rule out pneumothorax, pneumonia, and rib fractures which are treated with a chest tube, antibiotics, and analgesics, respectively. Furthermore, a CXR is useful in identifying an aortic dissection as a widened mediastinum may be present. If a PE is suspected, a CT pulmonary angiogram (gold standard) or a ventilation-perfusion scan (V/Q; used when CT is contraindicated such as in renal failure) may be used to diagnose and treatment entails either anticoagulation, thrombolytics, or catheter-directed thrombolysis. (2,8,19)
An esophagography may be useful in diagnosing esophageal etiologies such as an esophageal rupture which often warrants immediate surgical repair. Endoscopy and manometry are pertinent in diagnosing esophageal etiologies such as achalasia and esophageal spasms.
In conclusion, a thorough history, physical exam, and the use of predictive tools and diagnostic modalities are critical in diagnosing chest pain using an anatomical approach. Importantly, life-threatening causes should be ruled out and treated, since these etiologies progress quickly.
References
- Lentini S, Perrotta S. Aortic dissection with concomitant acute myocardial infarction: From diagnosis to management. J Emergencies, Trauma Shock. 2011 Apr;4(2):273–8.
- Vincent B. Young, William A. Kormos DAC. Blueprints Medicine. 6th ed. Wolters Kluwer; 2015. 416 p.
- Fass R, Achem SR. Noncardiac chest pain: Epidemiology, natural course and pathogenesis. J Neurogastroenterol Motil. 2011 Apr;17(2):110–23.
- Dries DJ. Chest Pain. Air Med J. 2016 May 1;35(3):107–10.
- Lenfant C. Chest pain of cardiac and noncardiac origin. Metabolism. 2010 Oct 1;59(SUPPL. 1):S41–6.
- McSweeney JC, Cody M, O’Sullivan P, Elberson K, Moser DK, Garvin BJ. Women’s Early Warning Symptoms of Acute Myocardial Infarction. Circulation. 2003 Nov 25;108(21):2619–23.
- Schey R, Villarreal A, Fass R. Noncardiac chest pain: Current treatment [Internet]. Vol. 3, Gastroenterology and Hepatology. Millenium Medical Publishing; 2007 [cited 2020 Aug 31]. p. 255–62. Available from: /pmc/articles/PMC3099272/?report=abstract
- Rushton S, Carman MJ. Chest Pain: If It Is Not the Heart, What Is It? Nurs Clin North Am. 2018 Sep 1;53(3):421–31.
- Lei WY, Wang JH, Wen SH, Yi CH, Hung JS, Liu TT, et al. Risk of acute myocardial infarction in patients with gastroesophageal reflux disease: A nationwide population-based study. PLoS One. 2017 Mar 1;12(3).
- Chen CH, Lin CL, Kao CH. Association between gastroesophageal reflux disease and coronary heart disease. Med (United States). 2016 Jul 1;95(27).
- Lau RK. Boerhaave syndrome. Pearls Pitfalls Emerg Radiol Var Other Difficult Diagnoses. 2010 Jan 1;125–7.
- Locke AB, Kirst N, Shultz CG. Diagnosis and Management of Generalized Anxiety Disorder and Panic Disorder in Adults. Am Fam Physician. 2015 May 1;91(9):617–24.
- Mcconaghy JR, Oza RS. Outpatient Diagnosis of Acute Chest Pain in Adults. Am Fam Physician. 2013 Feb 1;87(3):177–82.
- William E Cayley Jr. Diagnosing the cause of chest pain. Am Fam Physician. 2005;72(10):2012–21.
- Appleton C, Gillam L, Koulogiannis K. Cardiac Tamponade. Cardiol Clin. 2017 Nov 1;35(4):525–37.
- Cicala S, De Simone G, Roman MJ, Best LG, Lee ET, Wang W, et al. Prevalence and prognostic significance of wall-motion abnormalities in adults without clinically recognized cardiovascular disease: The strong heart study. Circulation. 2007 Jul 10;116(2):143–50.
- Hussain N, Parker MW, Henzlova MJ, Duvall WL. Stress-first Myocardial Perfusion Imaging. Cardiol Clin. 2016 Feb 1;34(1):59–67.
- Silaschi M, Byrne J, Wendler O. Aortic dissection: Medical, interventional and surgical management. Heart. 2017 Jan 1;103(1):78–87.
- Abou Ali A, Avgerinos ED, Chaer RA. Catheter-Directed Thrombolysis of Pulmonary Embolism. Curr Manag Venous Dis. 2018 Nov 25;389–403.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.