An Approach To
Chronic obstructive pulmonary disease (COPD)
Alexander Ni1
Published online: 19 October 2020
1Faculty of Medicine, McGill University, Montreal, QC, Canada
Corresponding Author: Alexander Ni, email alexander.ni@mail.mcgill.ca
Abstract
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the world, affecting developing and developed countries alike. Recent advancements in medical and interventional therapies have led to decreasing overall mortality rates in the last decade. Despite these improvements, COPD continues to cause significant morbidity and lost productivity. This review summarizes the pathophysiology of COPD and the work-up of a patient with suspected COPD, emphasizing pulmonary function testing, an approach to treatment, and the management of COPD exacerbations.
Tags: Chronic obstructive pulmonary disease (COPD), emphysema, chronic bronchitis, spirometry, COPD exacerbation, alpha-1-antitrypsin deficiency
Question
A 52-year-old man presents to his family physician with a 6-month history of worsening dyspnea with less and less exertion. He also complains of clear productive cough for the last 3 months. He was hospitalized last year for community acquired pneumonia. Past medical history is remarkable for hypertension, for which he takes perindopril and indapamide. He has smoked 1 pack of cigarettes daily for the last 30 years. On examination, his oxygen saturation is 93%. He appears fatigued. There is no accessory respiratory muscle use. There are bibasilar crackles and diffuse expiratory wheezes on auscultation. Laboratory results are shown below:
Complete blood count
Hemoglobin: 153 g/L (normal 130-170 g/L)
Hematocrit: 0.44 (normal 0.38-0.50)
Leukocyte count: 9.4 × 109 cells/L (normal 3.5-10.5 × 109 cells/L)
Platelet count: 245 × 109 cells/L (normal 130-380 × 109 cells/L)
Venous blood gas
pH: 7.41 (normal 7.34-7.45)
pCO2: 62 mmHg (normal 32-45 mmHg)
HCO3: 33 mmol/L (normal 22-27 mmol/L)
Lactate: 1.4 mmol/L (normal 0.5-2.5 mmol/L)
Assuming the most likely diagnosis, which of the following therapies has been shown to improve survival?
a) Daily oral azithromycin
b) Pulmonary rehabilitation
c) Inhaled budesonide/formoterol twice daily
d) Long-term supplemental oxygen
e) Daily inhaled tiotropium
Answer
D. The patient's clinical presentation of chronic progressive dyspnea with productive cough raises suspicion for chronic obstructive pulmonary disease (COPD), especially given his heavy smoking history. The recent hospitalization for pneumonia also suggests a possible COPD exacerbation. Laboratory investigations reveal respiratory acidosis with appropriate metabolic compensation, suggesting chronic CO2 retention. In COPD patients, long-term home oxygen is one of the few interventions shown to improve survival and slow disease progression. The other options have not been shown to reduce mortality. Pulmonary rehabilitation and inhaled antimuscarinic or corticosteroid/beta-agonist therapy improve symptoms and quality of life. Azithromycin has recently been shown to reduce the frequency and severity of COPD exacerbations.
Definition, risk factors, and pathophysiology
COPD is a clinical syndrome characterized by poorly reversible expiratory airflow limitation (1-3). This disease is the fifth leading cause of death and the second leading reason for hospitalization in Canada (4,5). Worldwide, over 3 million deaths were due to COPD in 2017 (1-3). Most patients with COPD present between 40 and 60 years of age (1-3). Cigarette smoking remains the predominant risk factor, although other inhalational exposures, such as those from combusted biomass, play a significant role (1-3,6,7). There are also genetic syndromes which confer an independent risk, particularly alpha-1-antitrypsin deficiency. A recent study from Québec has identified only the second genetic variant causing heritable emphysema (8).
Historically, COPD has been divided into two distinct clinical entities based on its most common etiologies (Table 1). Emphysema is a pathological term for alveolar parenchymal damage, leading to irreversible airspace dilation and hyperinflation (1-3). These patients are dubbed "pink puffers," since dyspnea is the predominant symptom and hypoxemia and hypercapnia occur later (1-3).
| Table 1: Comparison between emphysema and chronic bronchitis | Emphysema | Chronic bronchitis |
| Definition | Pathological: alveolar parenchymal destruction and irreversible airspace dilatation | Clinical: Productive cough on most days for > 3 months a year for > 2 years. |
| Pathogenesis | Imbalance between proteases and anti-proteases leads to destruction of lung parenchyma | Airway irritation leading to mucus gland hypertrophy/hyperplasia, mucus overproduction, and small airway clogging |
| Pathophysiology |
|
|
| Clinical features | "Pink puffer"
|
"Blue bloater"
|
Chronic bronchitis is defined clinically as "productive cough on most days for at least three months a year for two consecutive years" (1-3). Small airway obstruction is the hallmark, causing expiratory airflow limitation. The resulting alveolar hypoventilation leads to early hypoxemia and hypercapnia. Thus, these patients are called "blue bloaters," referring to early cyanosis and the frequent association with metabolic syndrome (1-3).
Clinically, significant overlap exists between emphysema and chronic bronchitis, since both are mainly caused by smoking (1-3,7). There has also been further characterization of an asthma-COPD overlap, since many patients exhibit features of both (1-3,9).
COPD frequently exhibits extra-pulmonary manifestations, notably sarcopenia and malnutrition (1-3). There are also many associated systemic co-morbidities contributing to worse outcomes, such as cardiovascular disease, metabolic syndrome, osteoporosis, depression/anxiety, gastro-esophageal reflux (GERD), and lung cancer (1-3,10). COPD may increase the risk for these conditions, particularly lung cancer (1-3,10).
Initial Approach
The history and physical examination are key to diagnosing COPD. Obtaining a detailed exposure history is critical, particularly for smoking and occupational exposures. The three core symptoms of COPD are dyspnea, cough, and sputum production, with progressive exertional dyspnea being the most common presenting feature (1-3). Physical examination is usually normal in early disease (11). With increasing severity, there may be signs of hyperinflation (hyper-resonant percussion and increased antero posterior chest diameter, known colloquially as a "barrel-chest") and hypoxemia (peripheral cyanosis) (11). Patients may display pursed lip breathing and accessory muscle use to compensate for dyspnea (11). Auscultation may reveal signs of small airway obstruction (11). Importantly, clubbing is rarely seen in COPD and should raise suspicion for another pulmonary pathology, especially malignancy (1-3,11).
1) Spirometry
Dynamic airflow testing should be performed whenever COPD is suspected. Flow volume loops often show an expiratory "scooping" characteristic of airflow obstruction (Figure 1) (1-3,12). Peak expiratory flow rate (PEF), 1-second forced expiration volume (FEV1), and forced vital capacity (FVC) all decrease in COPD (1-3). However, FEV1 is impaired more than FVC. Thus, measuring the FEV1/FVC ratio is critical; a ratio below 0.70 is diagnostic for obstructive lung disease (1-3). To differentiate COPD from asthma, the FEV1/FVC ratio should be repeated after administration of a bronchodilator, since non-reversible obstruction is more consistent with COPD (1-3).
Patients can be classified by severity based on the percent predicted FEV1 for age according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging system. Further investigations are strongly suggested when FEV1 is < 50% predicted (GOLD ≥ 3). (1-3) For patients with stage ≤ 2, a case-by-case approach is recommended (1-3).
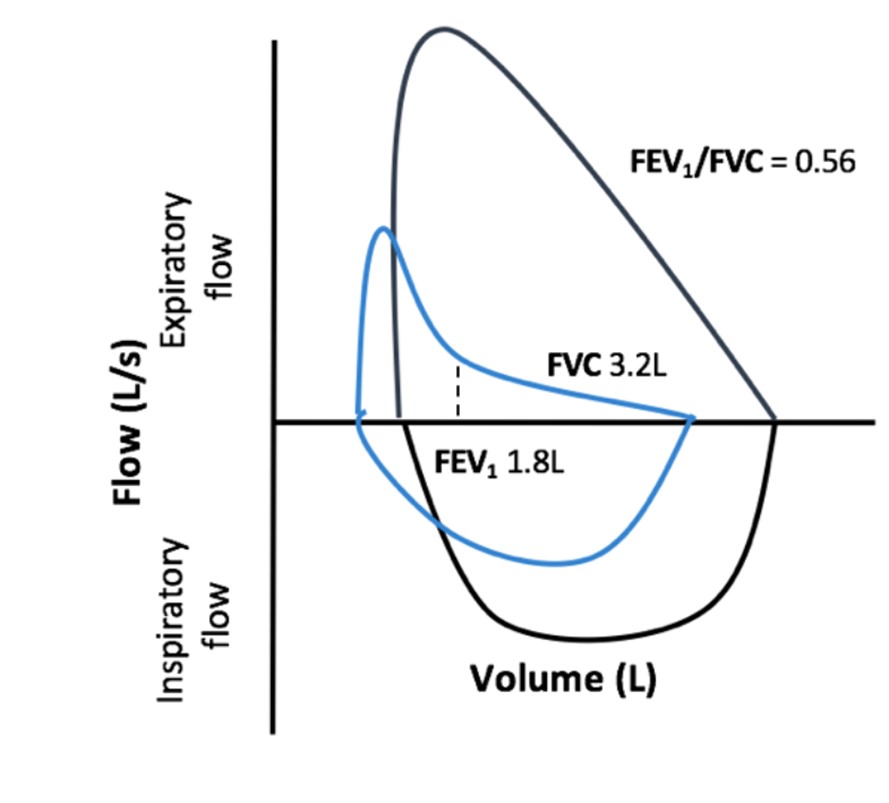
Adapted from Witt, L. #86: COPD: Diagnosis, treatment, PFTs, and nihilism. The Curbsiders, episode 86, March 18, 2018, http://thecurbsiders.com/podcast/86-copd-diagnosis-treatment-pfts-nihilism
2) Functional assessment
Grading of patients' symptoms can be accomplished using the modified Medical Research Council (mMRC) dyspnea scale. A score of 2 or greater (needing to stop for breath when walking at one's usual pace on level ground) indicates significant functional impairment (13). The COPD assessment test (CAT) is another available questionnaire to help determine the impact of the disease on patients' daily life. Recent studies have put forward the BODE index as a useful prognostic tool to predict 5-year survival (2,3). This index combines four variables: the body mass index (BMI), % predicted FEV1, mMRC score, and 6-minute walk distance (available at https://www.mdcalc.com/bode-index-copd-survival).
3) Plethysmography and diffusion capacity
Determining lung volumes can be useful in COPD with reduced FVC to assess the degree of hyperinflation (1-3). Increased residual volume (RV) without increased total lung capacity (TLC) indicates air trapping without hyperinflation, which is seen in early disease (1-3). As the airflow obstruction and parenchymal damage worsen, functional residual capacity (FRC) and TLC increase, suggesting hyperinflation (1-3).
Diffusion capacity can be assessed in patients with severe symptoms to quantify parenchymal damage (2,3). The diffusion capacity of carbon monoxide (DLCO) is a complex metric influenced by the gas exchange surface area, the diffusion barrier, and the pulmonary vascular supply (2,3). In COPD, decreased DLCO suggests decreased alveolar surface area, although other conditions such as interstitial lung disease and pulmonary vascular pathology need to be excluded (2,3).
4) Chest imaging
Chest imaging can help exclude other causes of dyspnea, such as pneumonia, congestive heart failure or malignancy, as well as other causes of decreased DLCO as mentioned above (1-3). Features suggestive of COPD include a flattened diaphragm and increased lung field translucency, which indicate hyperinflation and parenchymal damage respectively (1-3,14). Emphysema secondary to smoking is usually found in the upper lobes due to decreased perfusion (14). Lower lobe predominant emphysema is unusual and should raise suspicion for alpha-1-antitrypsin deficiency (2,3,14,15). Recently, chest CT has been increasingly employed to identify specific phenotypes of COPD at risk of severe disease or complications (2,3,14).
5) Co-morbidities
As described above, COPD can display extra-pulmonary manifestations and is frequently associated with other systemic diseases (1-3, 10). It is essential to assess for these co-morbidities, since they can lead to worsened outcomes and decreased overall quality of life. A complete blood count should be ordered to evaluate for anemia. Heart failure is an independent predictor of mortality in COPD with an annual incidence between 3-4% (1-3,10). Arrhythmias are also common in COPD, particularly atrial fibrillation, and more rarely, multifocal atrial tachycardia (1-3,10). More than 30% of COPD patients exhibit metabolic syndrome (1,16). Screening for lung cancer should be performed according to local guidelines (1-3,10). Under-diagnosed co-morbidities include osteoporosis and depression/anxiety, which may benefit from pulmonary rehabilitation (1,10). Apart from specific exceptions beyond the scope of this review, the treatment of co-morbidities is largely unchanged by the presence of COPD (1-3,10).
Beyond the Initial Approach
1) Basics of COPD management
a) Non-pharmacological measures
Smoking cessation is crucial for any smoker with COPD, since it is one of two measures proven to improve survival (1-3). Long-term home oxygen is the other therapy shown to reduce mortality but only for significantly hypoxemic patients with SpO2 < 88%, PaO2 < 55 mmHg, polycythemia, or clinical evidence of pulmonary hypertension (1-3). Pulmonary rehabilitation programs can be implemented to increase exercise tolerance -particularly if there is a history of exacerbations (1-3). It is also important to vaccinate all COPD patients for common respiratory pathogens such as influenza and Streptococcus pneumoniae to reduce the risk of exacerbations (1-3).
b) Pharmacological therapy
GOLD provides a stepwise approach that is summarized in Flowchart 1. Long acting muscarinic antagonists (LAMA) should be the initial therapy for all patients with short-acting beta-2 agonists (SABA) available as rescue therapy (1-3). The decision to add medications is based on the patient's symptoms, pulmonary function tests, and number of exacerbations (1-3). The next recommended agent is a combination LAMA and long-acting beta-2 agonist (LABA) (1-3). Inhaled corticosteroids (ICS) can also be added, especially if there are concomitant asthmatic features (2,3). Assessment of the blood eosinophil count may help identify patients who would respond to ICS, with > 300 cells/uL predictive of treatment benefit (1,2). ICS are frequently prescribed as an ICS/LABA combo. Triple therapy (ICS/LAMA/LABA) is usually reserved for patients with frequent exacerbations (> 2/year requiring hospitalization) (1-3). For patients with symptoms refractory to triple therapy, fourth-line options include azithromycin or the phosphodiesterase-4 inhibitor roflumilast, both of which may reduce the frequency of exacerbations (2,3). Importantly, none of the above therapies have been shown to improve survival (1-3).
c) Interventional techniques
Surgical techniques to correct hyperinflation may be considered when pharmacological therapy fails to control symptoms. Reduction in lung volumes increases efficiency of respiratory muscles and reduces ventilation-to-perfusion mismatch (1,17). Lung volume reduction surgery (LVRS) is an open surgical procedure that has been shown to improve survival in the subset of patients with severe upper-lobe emphysema and low baseline exercise capacity (1,17,18). Lung transplant may be an option for patients with very severe COPD to improve functional capacity, but it has an unclear benefit on overall mortality (1).
Minimally invasive bronchoscopy-based techniques have been developed to palliate the high morbidity inherent to open surgical procedures (1,17). Endobronchial valve (EBV) insertion mimics LVRS by causing atelectasis of the most diseased lobe (1,17,19). Recent studies have shown modest improvement in pulmonary function testing and exercise capacity with EBVs, particularly for patients with heterogenous emphysema (1,17,19). Other approaches include endobronchial coils and sclerosing therapies; further discussion is beyond the scope of this review.
d) Palliative therapies
Despite recent advancements in management, patients with COPD invariably experience gradual functional decline. (1-3) Early use of therapies once reserved for the end-of-life may help improve overall quality of life (1). Patients with persistent dyspnea may benefit from opiates and non-pharmacological measures such as fans blowing air toward the face, neuromuscular electrical stimulation, and chest wall vibration (1). Malnutrition is frequently encountered in COPD given its heightened metabolic state; thus, nutritional assessment and supplementation is often warranted (1). Even without hypoxemia, home oxygen may help reduce dyspnea and anxiety (1).
2) COPD exacerbation
a) Definition and evaluation
A COPD exacerbation is clinically defined as a "period of increased symptoms with or without concomitant features of an upper respiratory tract infection", which may range from mildly increased symptoms to life-threatening attacks (1-3,20). Exacerbations are most commonly precipitated by respiratory tract infections, with up to 30% attributable to viruses and 30-50% to bacteria (1-3,20). Air pollutants and cold temperatures also contribute to their development (1-3,20).
During a COPD exacerbation, patients are at increased risk of myocardial infarction and pulmonary emboli, which need to be excluded on presentation (2,3,20). A chest radiograph is useful to detect concomitant bacterial pneumonia. If a component of congestive heart failure is suspected, a brain natriuretic peptide (BNP) level should be obtained. Venous and/or arterial blood gases can help assess the degree of hypoxemia and hypercapnia.
b) Pharmacological treatment
Short-acting bronchodilators and systemic corticosteroids are the cornerstones of treatment (1-3,20). SABAs are given every 1-2 hours, preferably via metered dose inhaler, and with clinical improvement are diminished to every 3-4 hours (2,3,20). If the response is inadequate, short-acting muscarinic antagonists (SAMAs) may be added (2,20). There is no consensus on the optimal dose and duration of corticosteroid therapy, although recent studies have shown that a 5-day course of oral prednisone is as effective as 10-day or longer courses (2,20). For severe exacerbations, steroids should be given intravenously. Antibiotics are often administered, especially with severe symptoms or signs of bacterial pneumonia, such as high fever or purulent sputum (1-3,20).
Oxygen therapy is crucial in COPD exacerbations due to the risk of severe hypoxemia (1-3,20). However, patients are prone to acute hypercapnia with supplemental oxygen, which is explained by three main factors (1-3,20). First, chronic CO2 retention blunts the hypercapnic respiratory drive, so hypoxemia becomes the main breathing stimulus (2,20). Thus, oxygen may cause central hypoventilation. Second, oxygen may reverse hypoxic vasoconstriction in poorly ventilated areas of the lung and worsen ventilation-to-perfusion mismatch (2,20). Third, deoxygenated blood has a higher affinity for CO2 (via the Haldane effect). By increasing oxygenation, CO2 may be unloaded by red blood cells in the peripheral circulation, precipitating hypercapnia (2,20). To summarize, oxygen therapy should maintain a conservative saturation value (between 88-92%, equivalent to PaO2 60-70 mmHg) due to an increased mortality risk if higher saturations are targeted (1-3,20).
c) Ventilatory support
Severe exacerbations may require various degrees of ventilatory support. Non invasive mechanical ventilation (NIV) is preferred over invasive ventilation given the risks of ventilator-acquired pneumonia and barotrauma/volutrauma with intubation (1,20). Indications for NIV include hypoxemia despite supplemental oxygenation, refractory hypercapnia (PaCO2 > 45 mmHg and pH < 7.35), persistently increased work of breathing, or respiratory muscle fatigue (1,20). NIV can be discontinued without weaning once 4 hours of unassisted breathing is tolerated (1,20). Invasive mechanical ventilation should be initiated if patients fail NIV, have decreased level of consciousness, or are hemodynamically unstable (1,20). Many past indications for invasive ventilation can now be successfully managed with NIV (1,20).
3) Alpha-1-antitrypsin deficiency (AATD)
Alpha-1-antitrypsin (AAT) is a multivalent serum protease inhibitor. When deficient, patients are prone to emphysema due to uninhibited protease activity in the lung parenchyma. AAT is encoded by the SERPINA1 gene ("Pi") on chromosome 14, with "M" being the normal allele and "Z" being the most common deficient allele (15). Pi*ZZ homozygotes are at highest risk for severe emphysema., whereas Pi*MZ heterozygotes are at risk of severe disease with smoking or other inhalational exposures (7,15). Features suggestive of AATD include disease onset below 40 years of age and lower lobe predominant emphysema (14,15). Patients are also at risk for liver cirrhosis because defective AAT accumulates and damages hepatocytes (15). Any suggestive feature should prompt measurement of the serum AAT because intravenous AAT augmentation can slow progression of emphysema (1-3,15).
Flow Chart 1: Approach to the patient with suspected COPD
Spirometry is the cornerstone for the diagnosis of COPD. Once a non-reversible airflow obstruction is confirmed, the decision to order further investigations will depend on the disease severity as defined by objective and subjective clinical scoring systems. It is important to remember that emphysema and chronic bronchitis can be present in the absence of airflow obstruction. Also, persistent non-reversible airflow obstruction can be found in some patients with a clear history of long-standing asthma. An inhaled long acting muscarinic antagonist (LAMA) should be first-line maintenance therapy with a short-acting beta-2 agonist (SABA) as a rescue agent. Further medications can be added according to the patient's response to initial therapy. All patients should receive regular follow-up and necessary non-pharmacological interventions to reduce the risk of exacerbations and disease progression. Long-term oxygen supplementation is indicated with any sign of severe and/or chronic hypoxemia to delay the development of pulmonary hypertension and right heart failure.
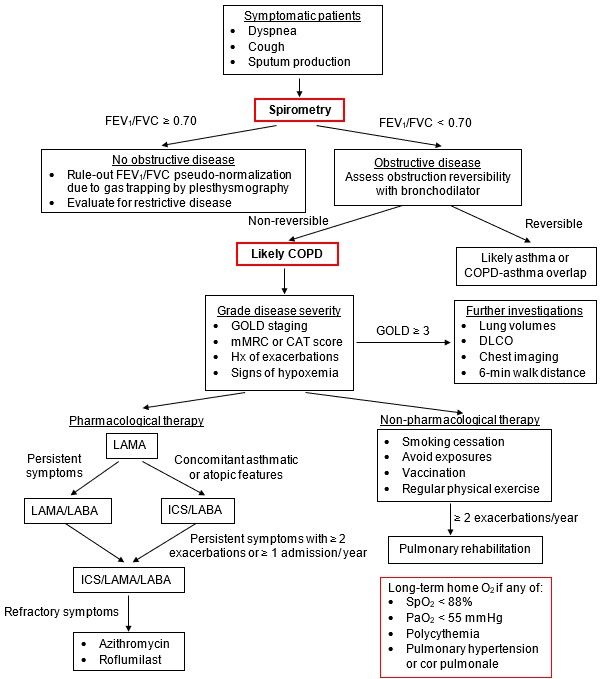
Adapted from GOLD 2020 Report and Celli BR, Wedzicha JA. Update on Clinical Aspects of Chronic Obstructive Pulmonary Disease. NEJM, 2019;381(13):1257-1266
References
- Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease, Global initiative for obstructive lung disease (GOLD) 2020 report. https://goldcopd.org/. Accessed 31 July 2020
- Celli BR, Wedzicha JA. Update on Clinical Aspects of Chronic Obstructive Pulmonary Disease. N Engl J Med 2019; 381(13):1257-1266.
- Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017; 389(10082): 1931-1940.
- Hospital stays in Canada. Canadian Institute for Health Information. 2020. https://www.cihi.ca/en/hospital-stays-in-canada. Accessed 31 July 2020.
- Leading causes of death, total population, by age group. Statistics Canada. 2018. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310039401. Accessed 31 July 2020.
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009; 374(9691): 733-743.
- Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015 ;385(9971): 899-909.
- Bossé Y et al. Early-onset emphysema in a large French-Canadian family: a genetic investigation. Lancet Respir Med. 2019; 7(5): 427-436.
- Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med. 2015 24;373(13):1241-9.
- Negewo NA, Gibson PG, McDonald VM. COPD and its comorbidities: Impact, measurement and mechanisms. Respirology. 2015;20(8):1160-71.
- Badgett RG et al. Can moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone? Am J Med. 1993; 94(2): 188-196.
- Witt, L. #86: COPD: Diagnosis, treatment, PFTs, and nihilism. The Curbsiders, episode 86, March 18, 2018. http://thecurbsiders.com/podcast/86-copd-diagnosis-treatment-pfts-nihilism. Accessed 31 July 2020.
- Stenton C. The MRC breathlessness scale. Occup Med (Lond). 2008; 58(3): 226-227.
- Lynch DA et al. CT-Definable Subtypes of Chronic Obstructive Pulmonary Disease: A Statement of the Fleischner Society. Radiology. 2015;277(1):192-205
- Strnad P, McElvaney NG, Lomas DA. Alpha1-Antitrypsin Deficiency. N Engl J Med. 2020; 382(15): 1443-1455.
- Cebron Lipovec N et al. The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: a systematic review. COPD. 2016;13(3):399-406.
- Shah PL et al. Lung volume reduction for emphysema. Lancet Respir Med. 2017 Feb;5(2):147-156.
- Fishman A et al; National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003; 348(21):2059-73
- Sciurba FC et al; VENT Study Research Group. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010; 363(13):1233-44.
- Ko FW et al. Acute exacerbation of COPD. Respirology. 2016;21(7):1152-1165.
- Ni, A, ed. "Block B: Respiration" from "Osler Notes". McGill Medical Students Society, 2019. Accessed 31 July 2020.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.