Approach to
Adrenal Mass
Erica Bitektine1
Published online: January 23, 2023
1Faculty of Medicine, McGill University, Montreal, Quebec, Canada
Corresponding Author: Erica Bitekine, email: erica.bitektine@mail.mcgill.ca
DOI: 10.26443/mjm.v21i1.1050
Abstract
Adrenal masses are a common incidental finding in imaging. Although most are benign, they should be thoroughly investigated to rule out malignancy and hormone hypersecretion. This article provides an approach to evaluating these adrenal masses, delving into the various differential diagnoses and special management considerations.
Tags: Adrenal mass, Adrenal incidentaloma, Imaging, Hormones, Endocrinology
Question
A 68-year-old male presents to the emergency room with right flank pain and is found to have a non-complicated obstructing kidney stone. On the non-contrast computed tomography (CT) scan of the abdomen, a left adrenal mass is also noted.
The patient is known for poorly controlled hypertension, type II diabetes mellitus, and spinal stenosis. He denies any personal or family history of adrenal cancer and recent malignancy. He does not have any recent weight loss, night sweats, or fatigue. He denies headaches, palpitations, and diaphoresis. Upon physical exam, he appears to have flank pain, proximal weakness on power testing, an elevated BMI, and 2-cm-wide red-purple lines (striae) on his thighs.
On the radiology report, the adrenal mass is described as unilateral left 2.4 cm x 3.6 cm, homogeneous with smooth border, and 6 HU (Hounsfield units). His complete blood count (CBC) is normal and electrolytes show hypokalemia at 3.3 mEq/L.
Once renal colic is resolved, which of the following investigations would you complete?
- 24-hour urine fractioned metanephrines and catecholamines
- Dexamethasone suppression test
- Aldosterone-renin ratio
- Dehydroepiandrotestosterone (DHEAS)
- CT scan of the abdomen with contrast
- Adrenal fine needle aspiration biopsy (FNA)
Which tests would you choose?
- a), b), and e)
- a), b), c), d), and e)
- a), b), e), and f)
- a), b), and c)
- e) and f)
Answer
4. is the correct answer. All adrenal masses should be investigated for pheochromocytoma with 24-hour urine fractioned metanephrines and catecholamines or plasma fractioned free metanephrines. Cushing’s syndrome should be evaluated using the dexamethasone suppression test or late-night salivary cortisol testing, given findings of Cushing’s upon examination (striae, high BMI, proximal weakness). Additionally, this patient is presenting with a history of hypertension and hypokalemia which suggests possible hyperaldosteronism and requires additional aldosterone-renin ratio testing. DHEAS testing is only warranted if the patient presents with symptoms of sex hormone excess, which is not the case here. The described imaging findings (smooth border, homogeneous, <4 cm, <10 HU) suggest that the mass is benign; therefore, a CT scan with contrast is not required at this stage. CT scans with contrast are used to measure contrast washout to differentiate benign and malignant masses when initial non-contrast CT features are suspicious (heterogeneous, irregular margins, necrosis, >10 HU attenuation, >4 cm in size). Lastly, FNA biopsies are not usually done as they do not differentiate between malignant and benign masses
Initial Approach
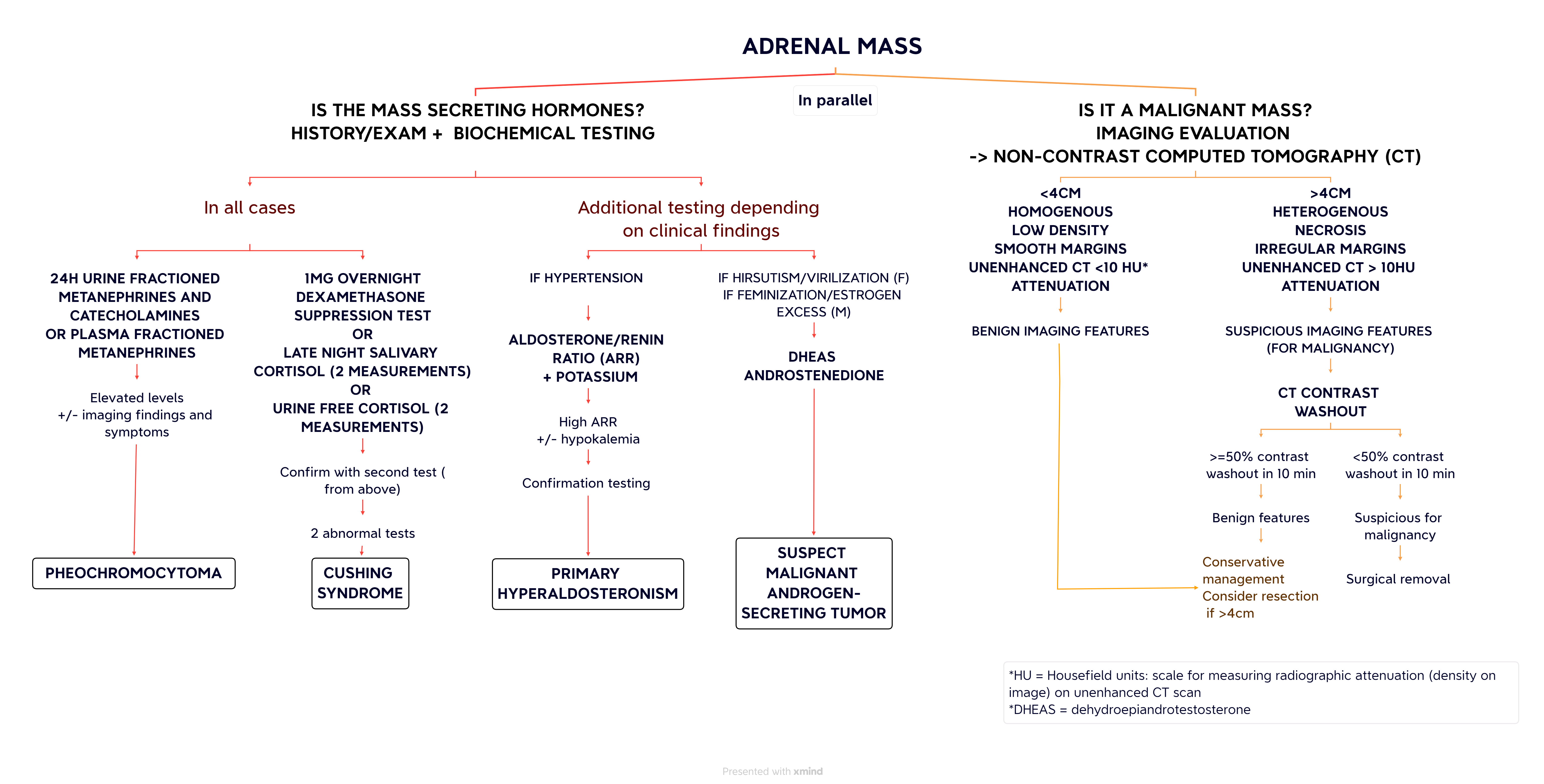
Adrenal masses are often accidentally discovered when a patient undergoes imaging studies for other medical reasons and are called incidentalomas. They have a mean prevalence of 2.3% at autopsy with a prevalence range from 1%-9%. A higher number of cases are found in older, white, obese, diabetic, and hypertensive people. (1)The majority of adrenal incidentalomas are benign non-functioning adenomas or lipomas. However, approximately 10%-15% are functional and result in the hypersecretion of cortisol (Cushing’s syndrome) or of other hormones (pheochromocytoma, hyperaldosteronism). Some cases can represent malignant primary tumours (2%-5%) or metastasis (1%-2.5%). (2) Incidentalomas require imaging and biochemical workups to rule out malignancy or hormone hypersecretion.
Evaluation for Malignancy
When evaluating a mass, malignancy should always be on the differential. Patients may report having a recent or active cancer, which could lead to possible metastasis of the adrenal gland. The most common metastatic sources are lung, breast, colon, stomach, lymphoma, melanoma, and renal cancers. (3) It is also important to ask about constitutional symptoms (weight loss, fever, night sweats, appetite loss, fatigue) and family history of malignancy.
A non-contrast abdominal CT scan is the main modality used to investigate adrenal masses for malignancy. The National Italian Study Group on adrenal tumours evaluated adrenal incidentaloma hospital records to retrospectively identify characteristics that suggest malignancy. Ascertained features include a larger size (>4 cm), irregular borders, heterogeneous masses, calcifications, and necrosis. (3-6) Additionally, on non-contrast CTs, radiographic attenuation of the mass is evaluated by Hounsfield units (HU). Malignant masses have higher HU (>10) as they absorb more X-rays than benign lipid-rich tissues, as was determined by Delivanis et al. (2018) in a retrospective cohort study, amongst others. (4,7) If the mass has a suspicious appearance on a non-contrast CT scan, an adrenal contrast washout CT protocol should be done. Adrenocortical carcinomas and other malignant masses commonly have decreased washout percentages (<40% relative enhancement loss at 15 minutes), whereas adenomas (benign masses) have higher washouts. (5) Pheochromocytomas can have similar characteristics to malignant masses on imaging. (8) Thus, it is important to rule out pheochromocytomas as described below.
If metastasis is suspected or the aforementioned imaging techniques are inconclusive, an FDG-PET (fluorodeoxyglucose positron emission tomography) scan can help assess for malignant features in indeterminate masses. In a prospective study by Guerin et al. (2017), MRIs were shown to aid in investigating the mass and distinguish pheochromocytomas. (9) Fine needle aspiration biopsy is not commonly done as it does not differentiate between benign and malignant adrenal tissue. However, it can help differentiate metastatic, extra-adrenal and primary adrenal malignancy, and allow to histological description of the tumour. If it is done, pheochromocytoma needs to be ruled out prior to avoid precipitating a pheochromocytoma crisis. (10,11)
Evaluation for Hormone Hypersecretion
Although most adrenal masses are non-functional, hormone hypersecretion needs to be ruled out by a thorough patient history, physical examination, and biochemical testing, as most patients are asymptomatic.
Cortisol Excess
ACTH (adrenocorticotropic hormone) independent cortisol excess or Cushing’s syndrome, is the most common presentation of a functioning adrenal mass. (4) In most cases, functional incidentalomas present with mild autonomous cortisol secretion (MACS), known as subclinical Cushing’s syndrome and have a prevalence of up to 20% amongst adrenal incidentalomas. (5) Patients with MACS lack the usual clinical findings associated with Cushing’s syndrome (menstrual changes, lethargy, bruising, hirsutism, round face, dorsal fat pad, striae, decreased libido, proximal muscle weakness, etc.) Instead, they may display the effects of cortisol hypersecretion in the form of hypertension, diabetes, dyslipidemia, obesity, atherosclerosis, and osteoporosis. (4,12). To assess for cortisol hypersecretion in an incidentaloma, the standard test is a 1 mg (low dose) overnight dexamethasone suppression test (DST). Alternatively, two measurements of late-night salivary cortisol or two measurements of urine-free cortisol can be done to assess for Cushing’s syndrome. If the first laboratory investigation is abnormal, another test, amongst the above-mentioned three tests, should be conducted to confirm the diagnosis of Cushing’s syndrome. (3,5)
Catecholamine Excess
Pheochromocytomas account for ~3% of incidentalomas and are another entity to rule out. (4) Although most pheochromocytomas are symptomatic, up to 15% of individuals may have normal blood pressure. (3) Clinical findings of pheochromocytoma catecholamine hypersecretion may include headache, sweating, tachycardia, palpitations, and hypertension. Before biochemical testing, certain medications (e.g., levodopa, adrenergic receptor agonists, TCAs, psychoactive agents, amphetamine, ethanol, etc.) should be discontinued if possible as they may cause false positives. Testing consists of 24-hour urine metanephrines and catecholamines or plasma-fractioned metanephrines. Metanephrines are products of catecholamine metabolism, and are, as a result, elevated in the context of catecholamine hypersecretion. (5)
Aldosterone Excess
Hypertensive patients with incidentalomas should also be screened for primary hyperaldosteronism (Conn’s syndrome). These patients may have unexplained hypokalemia and a family history of hyperaldosteronism or early-onset cerebrovascular accidents. Aldosteronomas are best tested by the plasma aldosterone-renin ratio. A high plasma aldosterone concentration to plasma renin activity ratio is abnormal and suggests primary hyperaldosteronism. (5,13) Certain medications, such as mineralocorticoid receptor antagonists, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers can affect test results. One of several downstream tests (oral sodium loading, saline infusion, fludrocortisone suppression test, captopril challenge) confirms the diagnosis. (14)
Sex Hormone Excess
Sex hormone-secreting adrenal tumors are rare and usually malignant. Sex hormone testing is only indicated if signs of unexpected virilization or excess estrogen are present without other symptoms of glucocorticoid excess. Testing is also done when there is a suspicion of adrenocortical carcinoma. (8) Symptoms of virilization include new onset hirsutism, acne, and deepening of voice in females. Estrogen hypersecretion in females results in irregular uterine bleeding and breast tenderness, while in males it is characterized by decreased libido, testicular atrophy, and gynecomastia. (5) Biochemical tests include dehydroepiandrotestosterone (DHEAS) and androstenedione.
Beyond the Initial Approach
This section details further considerations and management options.
Bilateral adrenal incidentalomas
Bilateral adrenal incidentalomas are found in 10%-15% of cases of incidentalomas and are estimated to have a prevalence of 0.3%-0.6% in the general population. (15) They can be found in the event of metastases, primary bilateral macronodular adrenal hyperplasia (PBMAH), bilateral cortical adenomas, congenital adrenal hyperplasia (CAH), amyloidosis, hemorrhage, granulomas, infiltrative disease, pheochromocytoma, Cushing’s syndrome, and primary hyperaldosteronism. (4,5)
The initial approach towards bilateral masses is as described above. However, bilateral masses are most likely to present with hormone hypersecretion compared to unilateral masses. Bilateral masses should be investigated for adrenal insufficiency if they appear to be hemorrhagic or infiltrative, as infiltration or destruction of adrenals can result in adrenocortical hyposecretion. (5) This is done by measuring fasting morning cortisol and ACTH levels, followed by an ACTH stimulation test. Adrenal insufficiency is present when cortisol increase is incomplete in response to ACTH injection. (16) With bilateral masses, late-onset CAH, specifically 21-hydroxylase deficiency, should be ruled out by measuring morning 17-hydroxyprogesterone levels. Very elevated levels are diagnostic, while moderate elevation requires ACTH stimulation testing to confirm the diagnosis. (5)
Management
Management of adrenal incidentalomas depends on biochemical and imaging findings. Masses >4 cm, those suspicious of malignancy and hyperfunctioning masses should be considered for resection. Adrenalectomies are usually conducted laparoscopically; however, open surgery is sometimes necessary. As per the latest European Society of Endocrinology guidelines, non-functioning benign-appearing masses below 4 cm, do not necessitate follow-up imaging. (5,17) On the other hand, the American Association of Clinical Endocrinologists recommends up to 5 years of radiological follow-up for all non-resected masses. (18) More recent European guidelines recommend that non-surgically removed masses >4 cm should be followed radiologically to monitor for stability, usually at 6-12 months. If there is a 5 mm and 20% size increase, surgical removal is recommended. (5,8) Repeat biochemical testing for hypersecretion should be done if a patient with initially non-functioning incidentaloma develops signs and symptoms of hormone hypersecretion. (5)
Of note, pheochromocytomas need to be promptly resected to avoid cardiovascular complications. Their removal is complicated by the risk of hypertensive crises, arrhythmias, and multiorgan failure. To prevent these complications, patients require pre-operative alpha-adrenergic blockade followed by beta-adrenergic blockade and subsequent high sodium diet. Beta-adrenergic blockade should never be given before alpha blockade in these patients. (19) As for subclinical Cushing’s syndrome-associated adrenal mass removal, it is important to test for adrenal insufficiency post-operatively using the ACTH stimulation test. Due to cortisol hypersecretion, the hypothalamic-pituitary-adrenal (HPA) axis is suppressed by negative feedback. When the cortisol-secreting mass is removed, the axis remains suppressed for some time resulting in a lack of adrenal stimulation by ACTH to release cortisol, leading to hypocorticolism. In the event of adrenocortical hypofunction, patients need to be given glucocorticoid replacement until the HPA axis recovers. (4)
References
- Barzon L, Sonino N, Fallo F, Palù G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. European Journal of Endocrinology. 2003;149(4):273–285
- Lacroix A. Clinical presentation and evaluation of adrenocortical tumors [Internet]. UpToDate. 2021 [cited 2022Dec24]. Available from: https://www.uptodate.com/contents/clinical-presentation-and-evaluation-of-adrenocortical-tumors?topicRef=153&source=see_link
- The Royal Australian College of general Practitioners. Incidental adrenal masses – a primary care approach [Internet]. Australian Family Physician. The Royal Australian College of general Practitioners; 2017 [cited 2022Dec24]. Available from: https://www.racgp.org.au/afp/2017/june/incidental-adrenal-masses-a-primary-care-approach
- Young WF, Kebebew E. Evaluation and management of the adrenal incidentaloma [Internet]. UpToDate. 2022 [cited 2022Dec24]. Available from: https://www.uptodate.com/contents/evaluation-and-management-of-the-adrenal-incidentaloma
- Sherlock M, Scarsbrook A, Abbas A, Fraser S, Limumpornpetch P, Dineen R, et al. Adrenal incidentaloma. Endocrine Reviews. 2020;41(6):775–820. https://doi.org/10.1210/endrev/bnaa008
- Angeli, A., Osella, G., Alì, A., & Terzolo, M. Adrenal incidentaloma: an overview of clinical and epidemiological data from the National Italian Study Group. Hormone research, 1997;47(4-6), 279–283. https://doi-org.proxy3.library.mcgill.ca/10.1159/000185477
- Delivanis DA, Bancos I, Atwell TD, Schmit GD, Eiken PW, Natt N, et al. Diagnostic performance of unenhanced computed tomography and 18F-fluorodeoxyglucose positron emission tomography in indeterminate adrenal tumours. Clinical Endocrinology. 2017;88(1):30–6. doi:10.1111/cen.13448
- Kapoor A, Morris T, Rebello R. Guidelines for the management of the incidentally discovered adrenal mass. Canadian Urological Association Journal. 2011;5(4):241–7. https://doi.org/10.5489/cuaj.11135
- Guerin C, Pattou F, Brunaud L, Lifante J-C, Mirallié E, Haissaguerre M, et al. Performance of 18F-FDG PET/CT in the characterization of adrenal masses in noncancer patients: A prospective study. The Journal of Clinical Endocrinology & Metabolism. 2017;102(7):2465–72. doi:10.1210/jc.2017-00254
- Sundin A, Hindié E, Avram AM, Tabarin A, Pacak K, Taïeb D. A clinical challenge: Endocrine and imaging investigations of Adrenal Masses. Journal of Nuclear Medicine. 2021Jul;62 (Supplement 2). https://doi.org/10.2967/jnumed.120.246066
- Moreira SG, Pow-Sang JM. Evaluation and management of Adrenal Masses. Cancer Control. 2002;9(4):326–34. https://doi.org/10.1177/107327480200900407
- Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of Adrenal Incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the study of adrenal tumors. European Journal of Endocrinology. 2016;175(2). https://doi.org/10.1530/EJE-16-0467
- Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism. 2016;101(5):1889–916. doi:10.1210/jc.2015-4061
- Cobb A, Aeddula NR. Primary Hyperaldosteronism. [Internet] StatPearls 2022 [cited 2022 Dec 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539779/
- Sweeney AT, Srivoleti P, Blake MA. Management of the patient with incidental bilateral adrenal nodules. Journal of Clinical and Translational Endocrinology: Case Reports. 2021;20:100082. https://doi.org/10.1016/j.jecr.2021.100082
- Bourdeau I, El Ghorayeb N, Gagnon N, Lacroix A. Management of endocrine disease: Differential Diagnosis, investigation and therapy of Bilateral Adrenal Incidentalomas. European Journal of Endocrinology. 2018;179(2). https://doi.org/10.1530/EJE-18-0296
- Fassnacht M , Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. European Journal of Endocrinology.2016;175(2):G1-G34.
- Zeiger MA, Thompson GB, Duh Q-Y, Hamrahian AH, Angelos P, Elaraj D, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the management of adrenal Incidentalomas: Executive summary of recommendations. Endocrine Practice. 2009;15(5):450–3. doi:10.4158/ep.15.5.450
- Patel D. Surgical approach to patients with pheochromocytoma. Gland Surgery. 2020;9(1):32–42. https://doi.org/10.21037/gs.2019.10.20

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .