Approach to
Approach to Ptosis
Nikhil S. Patil 1,
Adnan Pirbhai 2
Published online: October 2024
1Michael G. DeGroote School of Medicine, McMaster University, Hamilton, Ontario, Canada
2Department of Ophthalmology, McMaster University, Hamilton, Ontario, Canada
Corresponding Author: Adnan Pirbhai, email apirbhai@niagaraeyecarecentre.ca
DOI: 10.26443/mjm.v21i1.1038
Question
A 75-year-old male presents to the emergency room with a drooping left upper eyelid. Upon further questioning, he states that he feels his visual acuity has deteriorated in the left eye, but only in the dark. On testing his best corrected visual acuity is 20/25 in the right eye and 20/30 in the left eye. His pupils are equal, round, and reactive to light and accommodation. His intraocular pressures are 14mmHg bilaterally. His past medical history is significant for type 2 diabetes mellitus, hypertension, hypercholesterolemia, and obesity. He states that he first noticed his eyelid drooping this morning, and it does not get better or worse during the day. He also states that he has been experiencing some horizontal diplopia since this morning. Your clinical examination reveals no significant ocular misalignment, but extraocular movements of the patient’s left eye are limited in upward, downward, and inward gaze. His margin to reflex distance (distance between corneal light reflex and upper eyelid) is 2mm and he is unable to raise his eyelid more than 1mm, suggesting a decreased levator function. His ESR and CRP are within normal limits. At his 6-week follow-up appointment, his symptoms have almost entirely resolved. Given the patient’s clinical presentation, what is the most likely diagnosis?
- Third nerve palsy
- Myasthenia gravis
- Congenital ptosis
- Horner syndrome
- Aponeurotic ptosis
Tags: ptosis, droopy eyelid, third nerve palsy
Answer
A) Given the patient’s history and clinical examination, the most likely diagnosis is a third cranial nerve palsy. Furthermore, given his positive systemic risk factors (obesity, diabetes mellitus, hypertension, hypercholesterolemia) and absence of pupillary involvement, an ischemic third nerve palsy is favoured. Typically, ischemic third nerve palsies are self-limited and the patient can be scheduled for follow-up in 4-6 weeks with management of vascular risk factors. Pupillary involvement or lack of improvement at follow-up are indications for head imaging with CT angiography to rule out an aneurysm or other compressive etiologies. In this case, since the ptosis does not worsen as the day goes on, there is no fatigable ptosis on upgaze, and symptoms do not improve after rest, myasthenia gravis is unlikely. Congenital ptosis is also unlikely in this case as it would present in the first years of life. Horner syndrome would include miosis and facial anhidrosis alongside the ptosis. Aponeurotic ptosis (a result of normal aging) is a possible diagnosis; however, it is less likely in this patient given that the ptosis is not isolated and is accompanied by incomplete upward, downward, and inward gaze of the ipsilateral eye.
Initial Approach
Ptosis, also referred to as blepharoptosis, is defined as the lowering of the upper eyelid below the typical resting position. Generally, if the upper eyelid drops more than 1mm from the superior limbus, a diagnosis of ptosis can be made (1).
History taking should be focused on eliciting whether the ptosis is unilateral or bilateral, the time of onset, whether it was acute or progressive, whether it fluctuates during the day, pertinent family history, and any other ocular symptoms. Understanding the impact on daily functioning as well as the psychosocial impact are also relevant to the initial approach. It is particularly important to determine whether there are any other symptoms outside of the dropping eyelid for patients presenting with acute onset ptosis. Childhood photographs can help elicit the duration of ptosis. Past cancer history, risk factors, or constitutional symptoms should also be considered due to the possibility of an intracranial mass impeding on the innervation pathway or an eyelid mass physically weighing down the eyelid. In pediatric patients, head posture is another element to observe and ask caregivers about (1).
The clinical exam should focus on determining the margin-to-reflex distance (MRD), quantifying levator function, checking pupillary function, and assessing extraocular movements. There are three types of MRDs, however MRD 1 is typically the most clinically relevant, MRD 1 measures the corneal light reflex to the center of the upper eyelid margin and can quantify the degree of upper eyelid retraction. A normal MRD1 is greater than 2.5 mm, typically 4-5 mm (1,2). In patients with vertical strabismus, this measurement is not as useful. Levator function can be assessed by measuring the distance that the center of the upper eyelid margin travels between downgaze and upgaze, while the frontalis muscle is held in place. Normal levator function is 10mm or greater (1). As well, MRD 1 and levator function between the two eyes should be compared. It should be noted that proptosis in one eye may lead to an incorrect diagnosis of ptosis in the contralateral eye. Binocular Esterman visual field testing can help objectively determine the amount of visual compromise caused by ptosis. Depending on the suspected cause, specific investigations can be ordered such as serum acetylcholine receptor antibody levels, ice pack testing, CT or MRI of the head with or without angiography, or genetic testing (1). Lastly, eyelid palpation should be done in an effort to detect any palpable mass weighing the eyelid down.
Beyond the Initial Approach
This section outlines common causes of ptosis and is subdivided into two categories of ptosis: congenital and acquired (Figure 1). Treatment is also briefly discussed as a separate subsection.
Pseudoptosis
Some conditions can be mistaken for ptosis and this phenomenon is referred to as pseudoptosis. Dermatochalasis, a term for excessive redundant upper eyelid skin, can overlie the upper eyelid mimicking ptosis. Unilateral proptosis can cause the contralateral eyelid to appear ptotic due to ipsilateral lid retraction. Unilateral vertical strabismus can cause a ptotic appearance, specifically when the patient is looking down.
Congenital Ptosis
Patients presenting with a drooping eyelid within the first years of life are considered to have congenital ptosis. Although congenital ptosis is typically idiopathic, other causes including malignancy, autoimmune conditions, and hereditary disorders such as Duane syndrome or blepharophimosis syndrome should be ruled out (3,4). In congenital ptosis, there is fibrous and fatty infiltration of the levator palpebrae superioris with a poorly developed levator palpebrae superioris (3). Left untreated, congenital ptosis can lead to occlusion amblyopia (4).
Acquired Ptosis
Aponeurotic
The most common form of acquired ptosis, aponeurotic ptosis (also referred to as involutional ptosis), occurs due to the normal aging process and is a clinical diagnosis. The levator aponeurosis connects the levator palpebrae superioris to the upper eyelid tarsal plate. Disinsertion or dehiscence of the levator aponeurosis causes aponeurotic ptosis. Typically, levator function remains strong (5).
Myogenic
Myogenic ptosis is characterized by the weakening of the levator palpebrae superioris muscle, leading to ptosis. A common example is myasthenia gravis, an autoimmune condition characterized by elevated postsynaptic acetylcholine receptor antibodies at the neuromuscular junction leading to progressive muscle weakness (6). Notably, myasthenia gravis is sometimes classified as neurogenic ptosis given that its mechanism is localized to the neuromuscular junction. The most common presenting symptom of myasthenia gravis is ptosis, which can be accompanied by diplopia or blurred vision. These symptoms worsen with increased muscle use and therefore become worse as the day progresses and improve with rest (6,7). While ocular symptoms can be present in isolation, 50% of cases will progress to generalized myasthenia gravis (8). Determining whether the patient has difficulty with chewing or swallowing, proximal limb weakness, or respiratory muscle can also be useful when considering a diagnosis of myasthenia gravis. Ptosis improvement after the sleep test or ice test are also useful signs (6). These tests work as they reduce acetylcholine breakdown and therefore transiently improve ptosis caused by myasthenia gravis. Chronic progressive external ophthalmoplegia (a mitochondrial disorder), oculopharyngeal muscular dystrophy, and myotonic dystrophy are other examples of myogenic ptosis. Muscle biopsy, genetic testing, and looking at family photos are additional parts of the diagnostic approach.
Neurogenic
Neurogenic ptosis refers to ptosis that occurs as a result of dysfunction of the nerves innervating the eyelid muscles. The most common example is a third cranial nerve palsy, in which the clinical examination would also typically show the eye positioned inferior and lateral, limited adduction, infraduction, and/or supraduction of the eye, and occasionally a fixed dilated pupil. This is because the oculomotor nerve innervates the levator palpebrae superioris, superior rectus, inferior rectus, medial rectus, and inferior oblique muscles (9). The innervation to the pupil travels along the exterior portion of the oculomotor nerve, so pupil-sparing third nerve palsies typically have an ischemic cause that has only compromised the core of the oculomotor nerve (9). Notably, a third nerve palsy can be complete or partial, and it is possible for a partial third nerve palsy to present only with mild ptosis (10). Uncommonly, bilateral third nerve palsy can be present (11). If there is misalignment between the eyes, diplopia is a common chief complaint. The differential diagnosis for a third nerve palsy is vast, but can be generally stratified into compressive, vascular, neoplastic, or traumatic etiologies (10). Typically, ischemic third nerve palsies are self-resolving. Imaging such as a CT angiogram arch to vertex is often done to rule out a compressive vascular etiology which can have significant consequences. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are typically done as well to rule out giant cell arteritis. Another example of neurogenic ptosis is Horner syndrome which constitutes the characteristic triad of miosis, ptosis, and facial anhidrosis (12). Horner syndrome is always observed secondary to an underlying cause that disrupts the sympathetic innervation to the eye at the level of the head or neck. The causes can be stratified depending on the location of sympathetic innervation disruption has taken place: first-order neuron causes are typically intracranial, second-order neuron causes are in the thoracic region, and third-order neuron causes are typically near the cavernous sinus (13). Pseudo-enophthalmos, or the appearance that the eye has been posteriorly displaced in the orbit, may also be observed (13).
Traumatic
Traumatic ptosis includes a vast array of causes and occurs following a traumatic injury to the upper eyelid that disrupts the function of the levator palpebrae superioris. Traumatic ptosis can be further categorized into the neurogenic, myogenic, aponeurotic, or mechanical ptosis depending on the mechanism. For example, laceration of the levator muscle by a knife would be myogenic whereas excessive stretching of the eyelid leading to levator aponeurosis dehiscence would be aponeurotic.
Mechanical
Mechanical ptosis refers to drooping of the upper eyelid caused by the weight of the eyelid. A neoplastic eyelid mass, large hematoma in the eyelid, or excessive dermatochalasis are examples of mechanical ptosis.
Management
For ptosis associated with a treatable secondary cause, treatment of the underlying condition is the main objective. For patients with ptosis that does not have an underlying condition and is not bothersome to the patient, no treatment is necessary. Pediatric patients are followed more closely to monitor for signs of amblyopia (4). Levator muscle resection and frontalis suspension are common surgical procedures used for the treatment of ptosis (14).
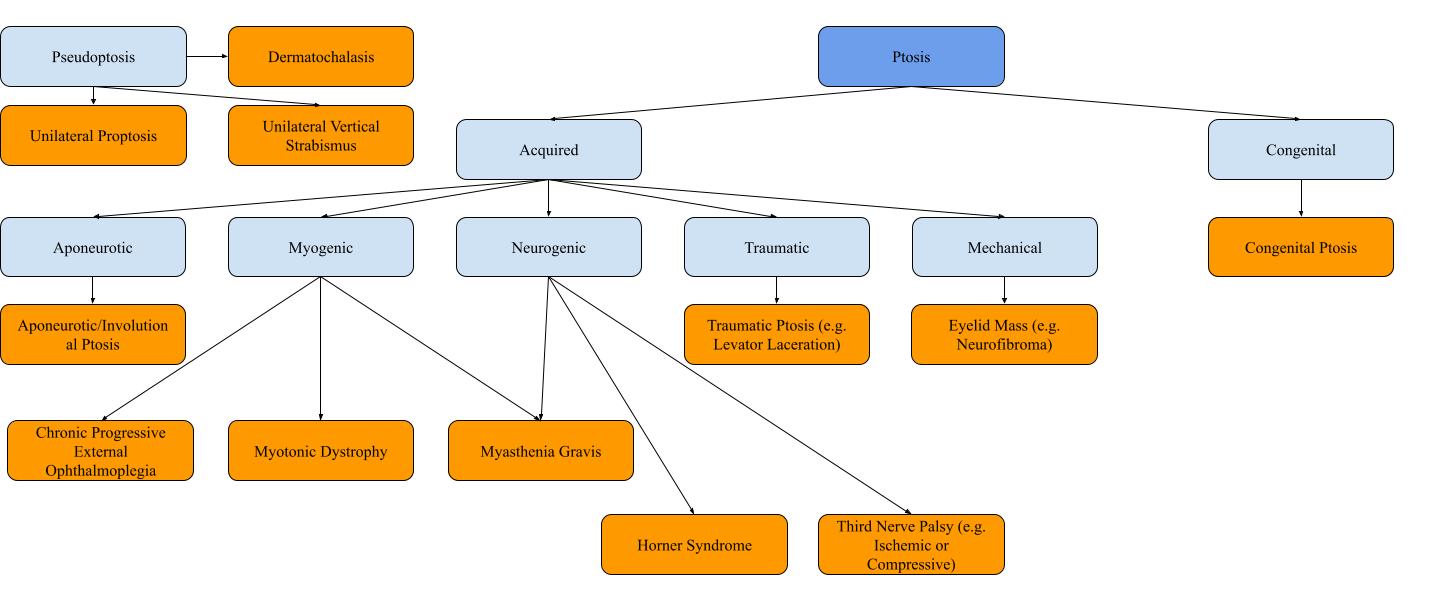
Ptosis diagnoses categorization flowchart.
References
- Finsterer J. Ptosis: Causes, Presentation, and Management. Aesthetic Plastic Surgery 2003 27:3 [Internet]. 2003 Aug 21 [cited 2022 Sep 25];27(3):193–204. Available from: https://link.springer.com/article/10.1007/s00266-003-0127-5
- Chen HC, Tzeng SS, Hsiao YC, Chen RF, Hung EC, Lee OK. Smartphone-Based Artificial Intelligence–Assisted Prediction for Eyelid Measurements: Algorithm Development and Observational Validation Study. JMIR Mhealth Uhealth [Internet]. 2021 Oct 1 [cited 2022 Sep 25];9(10). Available from: /pmc/articles/PMC8538024/
- Pavone P, Cho SY, Praticò AD, Falsaperla R, Ruggieri M, Jin DK. Ptosis in childhood: A clinical sign of several disorders: Case series reports and literature review. Medicine [Internet]. 2018 Sep 1 [cited 2022 Sep 25];97(36). Available from: /pmc/articles/PMC6133583/
- Marenco M, Macchi I, Macchi I, Galassi E, Massaro-Giordano M, Lambiase A. Clinical presentation and management of congenital ptosis. Clin Ophthalmol [Internet]. 2017 Feb 27 [cited 2022 Sep 25];11:453. Available from: /pmc/articles/PMC5338973/
- Plast Aesthet al, Floyd MT, Joon Kim H. More than meets the eye: a comprehensive review of blepharoptosis. Plast Aesthet Res [Internet]. 2021 Jan 8 [cited 2022 Sep 25];8:1. Available from: https://parjournal.net/article/view/3862
- Nair AG, Patil-Chhablani P, Venkatramani D v., Gandhi RA. Ocular myasthenia gravis: A review. Indian J Ophthalmol [Internet]. 2014 Oct 1 [cited 2022 Sep 25];62(10):985. Available from: /pmc/articles/PMC4278125/
- Toyka K v. Ptosis in myasthenia gravis: Extended fatigue and recovery bedside test. Neurology [Internet]. 2006 Oct 24 [cited 2022 Sep 25];67(8):1524–1524. Available from: https://n.neurology.org/content/67/8/1524
- Monsul NT, Patwa HS, Knorr AM, Lesser RL, Goldstein JM. The effect of prednisone on the progression from ocular to generalized myasthenia gravis. Journal of the neurological sciences. 2004 Feb 15;217(2):131-3
- Yanovitch T, Buckley E. Diagnosis and management of third nerve palsy. Curr Opin Ophthalmol [Internet]. 2007 Sep [cited 2022 Sep 25];18(5):373–8. Available from: https://pubmed.ncbi.nlm.nih.gov/17700229/
- Fang C, Leavitt JA, Hodge DO, Holmes JM, Mohney BG, Chen JJ. Incidence and Etiologies of Acquired Third Nerve Palsy Using a Population-Based Method. JAMA Ophthalmol [Internet]. 2017 Jan 1 [cited 2022 Sep 25];135(1):23. Available from: /pmc/articles/PMC5462106/
- Keane JR. Third Nerve Palsy: Analysis of 1400 Personally-examined Inpatients. Canadian Journal of Neurological Sciences [Internet]. 2010 Sep 1 [cited 2022 Sep 25];37(5):662–70. Available from: https://www.cambridge.org/core/journals/canadian-journal-of-neurological-sciences/article/third-nerve-palsy-analysis-of-1400-personallyexamined-inpatients/D077124C3F2B383734F5738F17A6D890
- Martin TJ. Horner Syndrome: A Clinical Review. ACS Chem Neurosci [Internet]. 2018 Feb 21 [cited 2022 Sep 25];9(2):177–86. Available from: https://pubs.acs.org/doi/abs/10.1021/acschemneuro.7b00405
- Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain [Internet]. 2015 Apr 10 [cited 2022 Sep 25];7:35–46. Available from: https://www.dovepress.com/horner-syndrome-clinical-perspectives-peer-reviewed-fulltext-article-EB
- Allard FD, Durairaj VD. Current Techniques in Surgical Correction of Congenital Ptosis. Middle East Afr J Ophthalmol [Internet]. 2010 [cited 2022 Sep 25];17(2):129. Available from: /pmc/articles/PMC2892127/

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.