Approach to
The Glaucoma Patient
Nikhil S. Patil1, David Dudok2
Published online: August 13, 2023
1Michael G. DeGroote School of Medicine, McMaster University, Hamilton, Ontario, Canada
2Department of Ophthalmology, McMaster University, Hamilton, Ontario, Canada
Corresponding Author: David Dudok, email: david.dudok@gmail.com
DOI: 10.26443/mjm.v21i1.1023
Tags: Intraocular pressure, Open-angle glaucoma, Closed-angle glaucoma, Ocular hypertension, Physiologic cupping
Question
A 62-year-old male presents to the ophthalmology service following a referral from his optometrist regarding dilated fundus exam abnormalities. The patient denies ocular symptoms and feels that his vision is normal. His past medical history is significant for type 2 diabetes mellitus, coronary artery disease, alcoholic liver disease, and no past ocular history. On exam, his best corrected visual acuity is 20/25 bilaterally. His intraocular pressure (IOP) is 16mmHg in the right eye and 12 mmHg in the left eye. He states that his pressures are “always around there” during visits to his optometrist’s office. His pupils are equal, round, and reactive to light and accommodation. Anterior segment examination is normal and the patient is phakic. On gonioscopy, there are open angles in both eyes and no synechiae or abnormal pigmentation. Dilated fundus exam shows a cup-to-disc ratio of 0.75 in both eyes and a right eye disc hemorrhage inferotemporally. There is peripapillary atrophy and inferoretinal neuroretinal rim thinning bilaterally, more severe in the right eye than the left. The macula is flat and there is no retinal tear or detachment. Visual field testing shows peripheral vision loss in the superior nasal quadrants (mean deviation -4-49 dB in the right eye and -2.01 dB in the left eye). Optical coherence tomography (OCT) of the macula is normal, although there is significant inferotemporal thinning of the retinal nerve fiber layer and ganglion cell complex thinning, more pronounced in the right eye compared to the left eye. Corneal thickness is within normal limits. The patient’s mother, grandmother, and aunt have glaucoma. What is the most likely diagnosis?
- Physiologic cupping
- Primary open-angle glaucoma
- Normal tension glaucoma
- Primary angle-closure glaucoma
- Secondary angle-closure glaucoma
Answer
C. Given the patient’s history and clinical examination findings, normal tension glaucoma is the most likely diagnosis. More specifically, findings such as bilateral nerve cupping, chronic visual loss, thinning retinal nerve fiber layer, normal IOP, and normal gonioscopy make this the most likely diagnosis. Notably, a diagnosis of glaucoma cannot be made with a single reading of IOP, and looking for progression through follow-up is of utmost importance. Physiologic cupping is not likely in this case given the glaucomatous vision loss and retinal nerve fiber layer changes. Primary open-angle glaucoma is less likely as well given that the IOP is within the normal range, although some sources consider normal tension glaucoma as a subtype of primary open-angle glaucoma. Primary and secondary angle-closure glaucoma are unlikely diagnoses in this case as gonioscopy showed open angles, and acute angle-closure would also present with significantly increased IOP.
Initial Approach
Intraocular pressure (IOP) is considered one of the “vital signs” of ophthalmology and is a key parameter to assess and follow in an initial approach to glaucoma. Normal mean IOP is 15 mmHg to 16 mmHg with a standard deviation of 3 mmHg (1). Elevated IOP is typically recognized as being the only modifiable risk factor for optic nerve damage, also known as glaucoma (1). The Early Manifest Glaucoma trial showed that a 25% reduction in IOP can decrease glaucoma progression by up to 50% (2). Beyond IOP, there are a number of other risk factors for the development of glaucoma including, but not limited to, age, ethnicity, and corneal thickness. Notably, one of the most important ophthalmological emergencies is acute angle-closure glaucoma, which if left untreated, can lead to rapid irreversible vision loss.
The “angle” of the eye refers to the space between the posterior cornea and the iris. This space contains the trabecular meshwork which allows aqueous humour to drain from the eye (3). More specifically, the aqueous humour clears into the trabecular meshwork, passes through Schlemm’s canal and the collector channels, drains into the episcleral venous plexus, and ultimately enters the venous system. Aqueous humour is produced by the ciliary body and fills the anterior chamber, thereby contributing to IOP.
There are important elements of the patient’s medical history that need to be elicited when a patient is presenting with an elevated IOP. Importantly, the patient should be asked about relevant past ocular history, including but not limited to past ophthalmic surgeries, uveitis, or trauma (4). Additionally, the clinician should determine the patient’s general past medical history, ethnicity, current medications, eyedrop use, as well as family history of glaucoma and other ocular conditions (4). A particular emphasis should be placed on any steroid and hypotensive medications alongside any history of diabetes mellitus, autoimmune conditions, sleep apnea, migraines, or Raynaud’s disease. The majority of patients with primary open-angle glaucoma, which is the most common form of glaucoma, are asymptomatic and incidentally diagnosed; however, it is important to ask about any self-perceived changes to patients’ visual acuity as well.
The most relevant clinical exam maneuver is the measurement of IOP. The gold standard of measurement is the Goldmann Applanation tonometer, although there are other tools such as the Tonopen and iCare which are typically used for screening (1, 5). The anterior segment slit lamp examination can provide important information regarding glaucoma etiology. For example, a Krukenberg spindle and transillumination (i.e. pigment dispersion syndrome), signs of pseudoexfoliation, or iris neovascularization secondary to diabetes can all provide vital information in diagnosis. The patient’s lens status is also an important consideration as cataracts can be a predisposing factor for glaucoma.
Next, gonioscopy is the gold standard for determining whether the angle is open or closed (1). It is important to note that myopic eyes are at increased risk of open-angle glaucoma and reduced risk for angle-closure glaucoma. Further, dilated fundus exam is essential, specifically in determining the optic nerve cup-to-disc ratio and inspecting for disc hemorrhages (1).
Contrasting the cup-to-disc ratio between eyes and examining the peripheral retina to are also key investigations for ruling out other pathologies that could contribute to elevated IOP, such as retinal detachment or uveitis. When analyzing the optic nerve head, a common “5 R’s” mnemonic can prove useful: observing the scleral ring to determine the limits of the optic disc size, identifying the rim size, inspecting the retinal nerve fiber layer, assessing for regions of peripapillary atrophy, and looking for disc or retinal hemorrhages.
Relevant investigations of IOP typically include optical coherence tomography (OCT) of the macula and optic nerve head, with a particular focus on the retinal nerve fiber layer and ganglion cell complex thickness. Ganglion cell thinning typically occurs first, followed by retinal nerve fiber layer thinning and, although the exact pathogenesis is not well established, oxidative stress is hypothesized to play a role (6). Visual field loss can be inversely correlated to the location of thinning (7). Visual field testing is insurmountably important in quantifying vision loss and is typically done in an outpatient setting. Data obtained from visual field testing can be cross-referenced with OCT retinal nerve fiber layer changes. Note that a thinner central corneal thickness (CCT) is an independent risk factor for glaucoma, so measuring corneal thickness is typically done with pachymetry in an outpatient setting as well. In some centers, ultrasound biomicroscopy or OCT can also be used to evaluate the anterior angle.
Beyond the Initial Approach
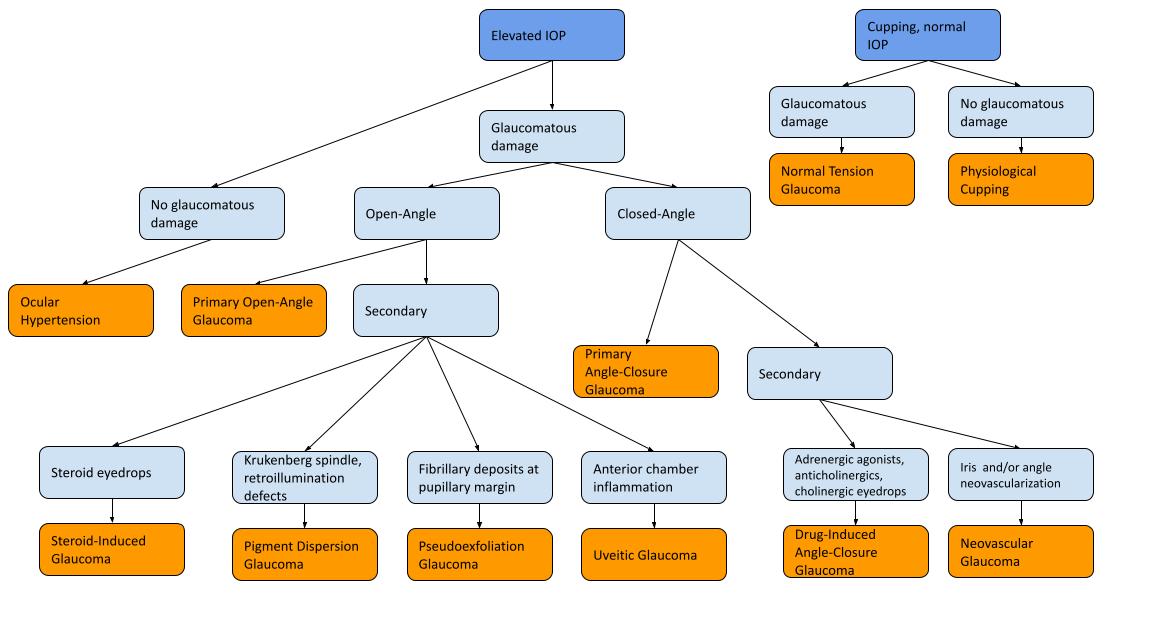
This section provides further information about open-angle and closed-angle glaucoma and is further divided into primary and secondary glaucoma. Primary glaucoma does not have a defined cause, whereas secondary glaucoma has a known underlying cause. Ocular hypertension and physiologic cupping are also discussed briefly in this section.
Ocular Hypertension and Physiologic Cupping
Ocular hypertension is defined as elevated IOP without glaucomatous optic nerve head changes or visual field changes, while physiologic cupping is the presence of an increased cup-to-disc ratio with normal IOP and visual fields. Neither of these diagnoses are pathological, although patients with ocular hypertension have an increased risk of developing glaucoma in the future and should be monitored closely (8). The Ocular Hypertension Treatment Study outlined several risk factors for progression of ocular hypertension to primary open-angle glaucoma including age, greater vertical and horizontal cup-disc ratio, pattern standard deviation, higher intraocular pressure, and thinner CCT (9). Many online calculators have been created based on the findings of this study to assess the risk of progression from ocular hypertension to glaucoma, which can be found on websites such as MDCalc (10).
Primary open-angle glaucoma
Primary open-angle glaucoma is the most common type of glaucoma in the Western World and is a problem of idiopathic reduced trabecular outflow (11). Risk factors include increased age, male sex, hypertension, diabetes mellitus, and African or European ethnicity (9,12). Primary open-angle glaucoma is a chronic condition characterized by peripheral vision loss and can be monitored using visual field testing with perimetry, cup-to-disc ratio measurement, and retinal nerve fiber layer thickness (12). Screening for primary open-angle glaucoma is crucial, with over 50% of glaucoma cases being hypothesized to remain undiagnosed (12).
Normal tension glaucoma
Normal tension glaucoma is a type of open-angle glaucoma where there is glaucomatous damage, but the IOP is within normal range. Some sources consider normal tension glaucoma as a subtype of primary open-angle glaucoma (11). Visual field defects, retinal nerve fiber layer thinning, or cupping of the nerve alongside normal IOP would raise suspicion for normal tension glaucoma (13). Although risk factors remain poorly understood, this condition is hypothesized to be related to vascular degeneration and neurodegenerative processes; associated conditions include Alzheimer’s dementia, migraines, Raynaud’s phenomenon, low blood pressure, and sleep apnea (13).
Secondary open-angle glaucoma
Secondary open-angle glaucoma includes several conditions; however, this subsection will focus on the most common ones. Pseudoexfoliation glaucoma is not fully understood but results in the production of white, fibrillary deposits in the anterior segment of the eye leading to clogging of the trabecular meshwork (14). Zonular weakness, higher IOP at presentation, and aggressive progression are characteristics of this disease (15). Pigmentary dispersion syndrome occurs secondary to pigment separating from the iris which, similar to the mechanism pseudoexfoliation glaucoma, prevents outflow through the trabecular meshwork (14). Pigmentary dispersion syndrome generally presents in younger patients with transillumination defects, Krukenberg spindles in the corneal endothelium, and significant pigmentation of the trabecular meshwork. In uveitic glaucoma, anterior chamber cells interfere with aqueous outflow. Notably, IOP is decreased in many types of uveitis (e.g. herpes simplex virus uveitis). Prolonged corticosteroid use plays a key role in cases of elevated IOP associated with uveitis (14). Steroid-induced glaucoma is an important secondary cause of glaucoma to make note of as many medical conditions are treated with systemic or topical steroids. Monitoring IOP after initiation of a topical steroid is essential, and IOP typically decreases shortly after the termination of steroid medication (14).
Primary angle-closure glaucoma
Primary angle closure (PAC) occurs when there is occlusion of the anterior angle of the eye preventing aqueous outflow through the trabecular meshwork without a predisposing secondary cause. Primary angle closure glaucoma (PACG) is defined as PAC with damage to the optic nerve. A primary angle closure suspect (PACS) is someone who has narrow angles as determined through gonioscopy. Hyperopia, female sex, and Inuit or Asian ethnicity are all risk factors for angle-closure glaucoma (16). Acute angle-closure leads to dramatic increases in IOP and can cause symptoms such as decreased visual acuity, halos, glare, headaches, nausea, vomiting, and a sensation of retro-orbital pressure. Chronic angle-closure refers to gradual narrowing of the angle leading to progressive glaucomatous damage (17). Patients with a narrow iridocorneal angle are at risk for periodic angle-closure attacks and can have chronic, progressive damage to their optic nerves as well. Over time, iridotrabecular synechiae may be observed (17).
Secondary angle-closure glaucoma
Secondary angle-closure can be further subdivided based on the presence or absence of pupillary block. Secondary pupillary block occurs when the iris moves posteriorly or the lens moves anteriorly and blocks communication between the posterior and anterior chamber, leading to a dramatic increase in IOP (16). Pupillary block can also be caused by posterior synechiae in the context of uveitis, or by iris and angle neovascularization in the context of diabetes mellitus (i.e. neovascular glaucoma). In neovascular glaucoma, the chronic contraction and proliferation of blood vessels leads to angle-closure glaucoma (17).
Drug-induced angle closure glaucoma with or without pupillary block is also possible. Adrenergic agonists, anticholinergics, and medications with anticholinergic side effects have been associated with angle-closure glaucoma with pupillary block (16). Sulfonamides, anticoagulants, and cholinergics have been associated with angle-closure glaucoma without pupillary block, mostly caused by anterior movement of the lens-iris diaphragm (16).
Management
Medical (ie. non-surgical) management of open-angle glaucoma is standard for the treatment of glaucoma, with the four most common classes of topical medications used being prostaglandin analogues, beta-blockers, cholinergic agonists, and carbonic anhydrase inhibitors (12). Given that the majority of patients are asymptomatic and do not feel any direct benefits of treatment, compliance remains one of the biggest challenges in glaucoma treatment (19). Selective laser trabeculoplasty to increase aqueous outflow has become commonplace in newly diagnosed open-angle glaucoma patients. In severe cases, surgical management options are considered including trabeculectomy, placement of a glaucoma drainage device, cataract surgery, or minimally invasive glaucoma surgery such as placement of intraocular stents (12). Angle-closure glaucoma is managed by resolving angle closure using laser peripheral iridotomy immediately or, in some cases, with clear-lens extraction (17, 20). Notably, a patient with unilateral acute angle closure has an increased likelihood of experiencing angle closure in the contralateral eye. Thus, peripheral iridotomy is typically performed in the contralateral eye as well. Prophylactic peripheral iridotomy was first explored by He et al. (2019) in the ZAP trial and is commonplace in many practices for patients with narrow anterior angles (21). Pilocarpine and oral carbonic anhydrase inhibitors can also be used for acute lowering of IOP.
Acknowledgements
N/A
Disclosure of Interest
The authors report no conflicts of interest.
References
- Nuzzi R, Marolo P, Nuzzi A. The Hub-and-Spoke Management of Glaucoma. Front Neurosci. 2020 Mar 17;14:180.
- Heijl, A., Leske, M. C., Bengtsson, B., Hyman, L., Bengtsson, B., Hussein, M., & Early Manifest Glaucoma Trial Group. (2002). Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Archives of ophthalmology, 120(10), 1268-1279.
- Nongpiur ME, Ku JYF, Aung T. Angle closure glaucoma: a mechanistic review. Curr Opin Ophthalmol [Internet]. 2011 Mar [cited 2022 Sep 27];22(2):96–101. Available from: https://pubmed.ncbi.nlm.nih.gov/21252671/
- McMonnies CW. Glaucoma history and risk factors. J Optom [Internet]. 2017 Apr 1 [cited 2022 Sep 27];10(2):71. Available from: /pmc/articles/PMC5383456/
- Paranhos A. J, Paranhos FRL, Prata J, Omi CA, Mello PAA, Bruce Shields M. Influence of keratometric readings on comparative intraocular pressure measurements with Goldmann, Tono-Pen, and noncontact tonometers. J Glaucoma [Internet]. 2000 [cited 2022 Sep 27];9(3):219–23. Available from: https://pubmed.ncbi.nlm.nih.gov/10877372/
- Izzotti A, Bagnis A, Saccà SC. The role of oxidative stress in glaucoma. Mutation Research/Reviews in Mutation Research. 2006 Mar 1;612(2):105-14.
- Wu H, De Boer JF, Chen L, Chen TC. Correlation of localized glaucomatous visual field defects and spectral domain optical coherence tomography retinal nerve fiber layer thinning using a modified structure–function map for OCT. Eye. 2015 Apr;29(4):525-33.
- Sun Y, Chen W, Wang N. [Diagnosis and treatment of ocular hypertension]. Zhonghua Yan Ke Za Zhi [Internet]. 2016 Jul 11 [cited 2022 Sep 27];52(7):542–6. Available from: https://pubmed.ncbi.nlm.nih.gov/27531115/
- Gordon, M. O., Beiser, J. A., Brandt, J. D., Heuer, D. K., Higginbotham, E. J., Johnson, C. A., ... & Ocular Hypertension Treatment Study Group. (2002). The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Archives of ophthalmology, 120(6), 714-720.
- Ocular hypertension treatment study (OHTS) calculator [Internet]. [cited 2023 Jun 5]. Available from: https://www.mdcalc.com/calc/10025/ocular-hypertension-treatment-study-ohts-calculator
- Kwon YH, Fingert JH, Kuehn MH, Alward WLM. Primary Open-Angle Glaucoma. N Engl J Med [Internet]. 2009 Mar 3 [cited 2022 Sep 27];360(11):1113. Available from: /pmc/articles/PMC3700399/
- Weinreb RN, Leung CKS, Crowston JG, Medeiros FA, Friedman DS, Wiggs JL, et al. Primary open-angle glaucoma. Nature Reviews Disease Primers 2016 2:1 [Internet]. 2016 Sep 22 [cited 2022 Sep 27];2(1):1–19. Available from: https://www.nature.com/articles/nrdp201667
- Killer HE, Pircher A. Normal tension glaucoma: review of current understanding and mechanisms of the pathogenesis. Eye [Internet]. 2018 May 1 [cited 2022 Sep 27];32(5):924. Available from: /pmc/articles/PMC5944657/
- Krishnadas R, Ramakrishnan R. Secondary Glaucomas: The Tasks Ahead. Community Eye Health [Internet]. 2001 [cited 2022 Sep 27];14(39):40. Available from: /pmc/articles/PMC1705934/
- Desai, M. A., & Lee, R. K. (2008). The medical and surgical management of pseudoexfoliation glaucoma. International ophthalmology clinics, 48(4), 95.
- Ah-Kee EY, Egong E, Shafi A, Lim LT, Yim JL. A review of drug-induced acute angle closure glaucoma for non-ophthalmologists. Qatar Med J [Internet]. 2015 Apr 1 [cited 2022 Sep 27];2015(1). Available from: /pmc/articles/PMC4614311/
- Wright C, Tawfik MA, Waisbourd M, Katz LJ. Primary angle-closure glaucoma: an update. Acta Ophthalmol [Internet]. 2016 May 1 [cited 2022 Sep 27];94(3):217–25. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/aos.12784
- Rodrigues GB, Abe RY, Zangalli C, Sodre SL, Donini FA, Costa DC, et al. Neovascular glaucoma: A review. Int J Retina Vitreous [Internet]. 2016 Dec 12 [cited 2022 Sep 27];2(1):1–10. Available from: https://journalretinavitreous.biomedcentral.com/articles/10.1186/s40942-016-0051-x
- Robin, A., & Grover, D. S. (2011). Compliance and adherence in glaucoma management. Indian journal of ophthalmology, 59(Suppl1), S93.
- Azuara-Blanco, A., Burr, J., Ramsay, C., Cooper, D., Foster, P. J., Friedman, D. S., ... & Norrie, J. (2016). Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. The Lancet, 388(10052), 1389-1397.
- He, M., Jiang, Y., Huang, S., Chang, D. S., Munoz, B., Aung, T., ... & Friedman, D. S. (2019). Laser peripheral iridotomy for the prevention of angle closure: a single-centre, randomised controlled trial. The Lancet, 393(10181), 1609-1618.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.